The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1
- PMID: 19931610
- PMCID: PMC3098384
- DOI: 10.1016/j.bbi.2009.11.006
The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1
Abstract
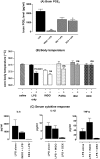
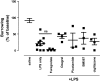
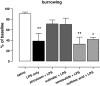
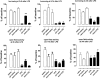
Systemic inflammation gives rise to metabolic and behavioural changes, largely mediated by pro-inflammatory cytokines and prostaglandin production (PGE(2)) at the blood-brain barrier. Despite numerous studies, the exact biological pathways that give rise to these changes remains elusive. This study investigated the mechanisms underlying immune-to-brain communication following systemic inflammation using various anti-inflammatory agents. Mice were pre-treated with selective cyclo-oxygenase (COX) inhibitors, thromboxane synthase inhibitors or dexamethasone, followed by intra-peritoneal injection of lipopolysaccharide (LPS). Changes in body temperature, open-field activity, and burrowing were assessed and mRNA and/or protein levels of inflammatory mediators measured in serum and brain. LPS-induced systemic inflammation resulted in behavioural changes and increased production of IL-6, IL-1beta and TNF-alpha, as well as PGE(2) in serum and brain. Indomethacin and ibuprofen reversed the effect of LPS on behaviour without changing peripheral or central IL-6, IL-1beta and TNF-alpha mRNA levels. In contrast, dexamethasone did not alter LPS-induced behavioural changes, despite complete inhibition of cytokine production. A selective COX-1 inhibitor, piroxicam, but not the selective COX-2 inhibitor, nimesulide, reversed the LPS-induced behavioural changes without affecting IL-6, IL-1beta and TNF-alpha protein expression levels in the periphery or mRNA levels in the hippocampus. Our results suggest that the acute LPS-induced changes in burrowing and open-field activity depend on COX-1. We further show that COX-1 is not responsible for the induction of brain IL-6, IL-1beta and TNF-alpha synthesis or LPS-induced hypothermia. Our results may have implications for novel therapeutic strategies to treat or prevent neurological diseases with an inflammatory component.
2009 Elsevier Inc. All rights reserved.
Figures
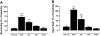
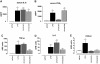
Comment in
-
Letter to the editor re: "The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1." by Teeling et al.Brain Behav Immun. 2011 Mar;25(3):584-5; author reply 586. doi: 10.1016/j.bbi.2010.11.015. Epub 2010 Dec 7. Brain Behav Immun. 2011. PMID: 21144893 No abstract available.
References
-
- Aisen P.S. Evaluation of selective COX-2 inhibitors for the treatment of Alzheimer’s disease. J. Pain Symptom Manage. 2002;23:S35–S40. - PubMed
-
- Blatteis C.M. The onset of fever: new insights into its mechanism. Prog. Brain Res. 2007;162:3–14. - PubMed
-
- Bluthe R.M., Laye S., Michaud B., Combe C., Dantzer R., Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci. 2000;12:4447–4456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

