Unimpaired trace classical eyeblink conditioning in Purkinje cell degeneration (pcd) mutant mice
- PMID: 19931625
- PMCID: PMC2843805
- DOI: 10.1016/j.nlm.2009.11.004
Unimpaired trace classical eyeblink conditioning in Purkinje cell degeneration (pcd) mutant mice
Abstract
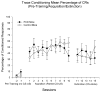
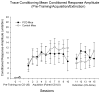
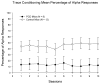
Young adult Purkinje cell degeneration (pcd) mutant mice, with complete loss of cerebellar cortical Purkinje cells, are impaired in delay eyeblink classical conditioning. In the delay paradigm, the conditioned stimulus (CS) overlaps and coterminates with the unconditioned stimulus (US), and the cerebellar cortex supports normal acquisition. The ability of pcd mutant mice to acquire trace eyeblink conditioning in which the CS and US do not overlap has not been explored. Recent evidence suggests that cerebellar cortex may not be necessary for trace eyeblink classical conditioning. Using a 500 ms trace paradigm for which forebrain structures are essential in mice, we assessed the performance of homozygous male pcd mutant mice and their littermates in acquisition and extinction. In contrast to results with delay conditioning, acquisition of trace conditioning was unimpaired in pcd mutant mice. Extinction to the CS alone did not differ between pcd and littermate control mice, and timing of the conditioned response was not altered by the absence of Purkinje cells during acquisition or extinction. The ability of pcd mutant mice to acquire and extinguish trace eyeblink conditioning at levels comparable to controls suggests that the cerebellar cortex is not a critical component of the neural circuitry underlying trace conditioning. Results indicate that the essential neural circuitry for trace eyeblink conditioning involves connectivity that bypasses cerebellar cortex.
2009 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice.J Neurosci. 1996 Apr 15;16(8):2829-38. doi: 10.1523/JNEUROSCI.16-08-02829.1996. J Neurosci. 1996. PMID: 8786457 Free PMC article.
-
Classical eyeblink conditioning in glutamate receptor subunit delta 2 mutant mice is impaired in the delay paradigm but not in the trace paradigm.Eur J Neurosci. 2001 Mar;13(6):1249-53. doi: 10.1046/j.0953-816x.2001.01488.x. Eur J Neurosci. 2001. PMID: 11285022
-
Model-Driven Analysis of Eyeblink Classical Conditioning Reveals the Underlying Structure of Cerebellar Plasticity and Neuronal Activity.IEEE Trans Neural Netw Learn Syst. 2017 Nov;28(11):2748-2762. doi: 10.1109/TNNLS.2016.2598190. IEEE Trans Neural Netw Learn Syst. 2017. PMID: 27608482
-
The Anatomy and Physiology of Eyeblink Classical Conditioning.Curr Top Behav Neurosci. 2018;37:297-323. doi: 10.1007/7854_2016_455. Curr Top Behav Neurosci. 2018. PMID: 28025812 Review.
-
Ontogenetic changes in the neural mechanisms of eyeblink conditioning.Integr Physiol Behav Sci. 2001 Jan-Mar;36(1):15-35. doi: 10.1007/BF02733945. Integr Physiol Behav Sci. 2001. PMID: 11484994 Review.
Cited by
-
Aging in the cerebellum and hippocampus and associated behaviors over the adult life span of CB6F1 mice.Neuroscience. 2013 Sep 5;247:335-50. doi: 10.1016/j.neuroscience.2013.06.002. Epub 2013 Jun 11. Neuroscience. 2013. PMID: 23764510 Free PMC article.
-
The Childhood-Onset Neurodegeneration with Cerebellar Atrophy (CONDCA) Disease Caused by AGTPBP1 Gene Mutations: The Purkinje Cell Degeneration Mouse as an Animal Model for the Study of this Human Disease.Biomedicines. 2021 Sep 4;9(9):1157. doi: 10.3390/biomedicines9091157. Biomedicines. 2021. PMID: 34572343 Free PMC article. Review.
-
Mutation-related differences in exploratory, spatial, and depressive-like behavior in pcd and Lurcher cerebellar mutant mice.Front Behav Neurosci. 2015 May 12;9:116. doi: 10.3389/fnbeh.2015.00116. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26029065 Free PMC article.
-
Timing correlations between cerebellar interpositus neuronal firing and classically conditioned eyelid responses in wild-type and Lurcher mice.Sci Rep. 2018 Jul 16;8(1):10697. doi: 10.1038/s41598-018-29000-w. Sci Rep. 2018. PMID: 30013234 Free PMC article.
-
Prefrontal control of cerebellum-dependent associative motor learning.Cerebellum. 2014 Feb;13(1):64-78. doi: 10.1007/s12311-013-0517-4. Cerebellum. 2014. PMID: 24013852
References
-
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. - PubMed
-
- Bao S, Chen L, Thompson RF. Classical eyeblink conditioning in two strains of mice: conditioned responses, sensitization, and spontaneous eyeblinks. Behavioral Neuroscience. 1998a;112:714–718. - PubMed
-
- Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Experimental Brain Research. 1994;100:469–483. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

