Smooth muscle phenotypic modulation is an early event in aortic aneurysms
- PMID: 19931668
- PMCID: PMC2956879
- DOI: 10.1016/j.jtcvs.2009.07.075
Smooth muscle phenotypic modulation is an early event in aortic aneurysms
Abstract
Objectives: Vascular smooth muscle cells can undergo profound changes in phenotype, defined by coordinated repression of smooth muscle cell marker genes and production of matrix metalloproteinases in response to injury. However, little is known of the role of smooth muscle cells in aortic aneurysms. We hypothesized that smooth muscle cells undergo phenotypic modulation early in the development of aortic aneurysms.
Methods: Abdominal aortas from C57B6 mice (n = 79) were perfused with elastase or saline (control) and harvested at 1, 3, 7, or 14 days. Aortas were analyzed by means of quantitative polymerase chain reaction and immunohistochemistry for smooth muscle cell marker genes, including SM22A, smooth muscle alpha-actin, and matrix metalloproteinases 2 and 9. In complimentary experiments human aneurysms (n = 10) and control aorta (n = 10) were harvested at the time of surgical intervention and analyzed.
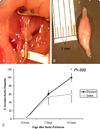
Results: By 14 days, aortic diameter was larger after elastase perfusion compared with control diameter (100% +/- 9.6% vs 59.5% +/- 18.9%, P = .0002). At 7 days, elastase-perfused mice had a 78% and 85% reduction in SM22 alpha and smooth muscle alpha-actin expression, respectively, compared with that seen in control animals well before aneurysms were present, and these values remained repressed at 14 days. Immunohistochemistry confirmed less SM22 alpha and smooth muscle alpha-actin in experimental aneurysms at 14 days in concert with increased matrix metalloproteinase 2 and 9 expression at 7 and 14 days. Similarly, human aneurysms had less SM22 alpha and smooth muscle alpha-actin and increased matrix metalloproteinase 2 and 9 staining, compared with control values, as determined by means of quantitative polymerase chain reaction.
Conclusions: Aneurysms demonstrate smooth muscle cell phenotypic modulation characterized by downregulation of smooth muscle cell marker genes and upregulation of matrix metalloproteinases. These events in experimental models occur before aneurysm formation. Targeting smooth muscle cells to a reparative phenotype might provide a novel therapy in the treatment of aortic aneurysms.
Figures





References
-
- Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584–588. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Kawai-Kowase K, Owens GK. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C59–C69. - PubMed
-
- Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–981. - PubMed
-
- Ailawadi G, Eliason JL, Roelofs KJ, et al. Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2004;24:2116–2122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

