Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production
- PMID: 19932078
- PMCID: PMC4073618
- DOI: 10.1016/j.abb.2009.11.019
Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production
Abstract
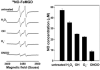
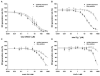
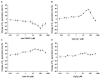
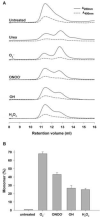
Inducible NOS (iNOS) is induced in diseases associated with inflammation and oxidative stress, and questions remain regarding its regulation. We demonstrate that reactive oxygen/nitrogen species (ROS/RNS) dose-dependently regulate iNOS function. Tetrahydrobiopterin (BH4)-replete iNOS was exposed to increasing concentrations of ROS/RNS and activity was measured with and without subsequent BH4 addition. Peroxynitrite (ONOO(-)) produced the greatest change in NO generation rate, approximately 95% decrease, and BH4 only partially restored this loss of activity. Superoxide (O2(.-)) greatly decreased NO generation, however, BH4 addition restored this activity. Hydroxyl radical ((.)OH) mildly decreases NO generation in a BH4-dependent manner. iNOS was resistant to H2O2 with only slightly decreased NO generation with up to millimolar concentrations. In contrast to the inhibition of NO generation, ROS enhanced O2(.-) production from iNOS, while ONOO(-) had the opposite effect. Thus, ROS promote reversible iNOS uncoupling, while ONOO(-) induces irreversible enzyme inactivation and decreases both NO and O2(.-) production.
2009 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

