Myoblast fusion: when it takes more to make one
- PMID: 19932206
- PMCID: PMC2854170
- DOI: 10.1016/j.ydbio.2009.10.024
Myoblast fusion: when it takes more to make one
Abstract
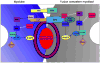
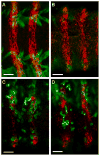
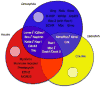
Cell-cell fusion is a crucial and highly regulated event in the genesis of both form and function of many tissues. One particular type of cell fusion, myoblast fusion, is a key cellular process that shapes the formation and repair of muscle. Despite its importance for human health, the mechanisms underlying this process are still not well understood. The purpose of this review is to highlight the recent literature pertaining to myoblast fusion and to focus on a comparison of these studies across several model systems, particularly the fly, zebrafish and mouse. Advances in technical analysis and imaging have allowed identification of new fusion genes and propelled further characterization of previously identified genes in each of these systems. Among the cellular steps identified as critical for myoblast fusion are migration, recognition, adhesion, membrane alignment and membrane pore formation and resolution. Importantly, striking new evidence indicates that orthologous genes govern several of these steps across these species. Taken together, comparisons across three model systems are illuminating a once elusive process, providing exciting new insights and a useful framework of genes and mechanisms.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures



References
-
- Artero RD, Castanon I, Baylies MK. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development. 2001;128:4251–64. - PubMed
-
- Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278:19266–71. - PubMed
-
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

