Characterization of WRSs2 and WRSs3, new second-generation virG(icsA)-based Shigella sonnei vaccine candidates with the potential for reduced reactogenicity
- PMID: 19932216
- PMCID: PMC2999844
- DOI: 10.1016/j.vaccine.2009.11.001
Characterization of WRSs2 and WRSs3, new second-generation virG(icsA)-based Shigella sonnei vaccine candidates with the potential for reduced reactogenicity
Abstract
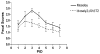
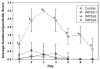
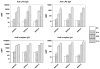
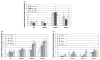
Live, attenuated Shigella vaccine candidates, such as Shigella sonnei strain WRSS1, Shigella flexneri 2a strain SC602, and Shigella dysenteriae 1 strain WRSd1, are attenuated principally by the loss of the VirG(IcsA) protein. These candidates have proven to be safe and immunogenic in volunteer trials and in one study, efficacious against shigellosis. One drawback of these candidate vaccines has been the reactogenic symptoms of fever and diarrhea experienced by the volunteers, that increased in a dose-dependent manner. New, second-generation virG(icsA)-based S. sonnei vaccine candidates, WRSs2 and WRSs3, are expected to be less reactogenic while retaining the ability to generate protective levels of immunogenicity seen with WRSS1. Besides the loss of VirG(IcsA), WRSs2 and WRSs3 also lack plasmid-encoded enterotoxin ShET2-1 and its paralog ShET2-2. WRSs3 further lacks MsbB2 that reduces the endotoxicity of the lipid A portion of the bacterial LPS. Studies in cell cultures and in gnotobiotic piglets demonstrate that WRSs2 and WRSs3 have the potential to cause less diarrhea due to loss of ShET2-1 and ShET2-2 as well as alleviate febrile symptoms by loss of MsbB2. In guinea pigs, WRSs2 and WRSs3 were as safe, immunogenic and efficacious as WRSS1.
Published by Elsevier Ltd.
Figures
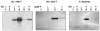

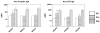
References
-
- Niyogi SK. Increasing antimicrobial resistance—an emerging problem in the treatment of shigellosis. Clinical Microbiology and Infection. 2007 December;13(12):1141–3. - PubMed
-
- Morpeth SC, Thielman NM. Diarrhea in patients with AIDS. Current Treatment Options in Gastroenterology. 2006 February;9(1):23–37. - PubMed
-
- Iwalokun BA, Gbenle GO, Smith SI, Ogunledun A, Akinsinde KA, Omonigbehin EA. Epidemiology of shigellosis in Lagos, Nigeria: trends in antimicrobial resistance. Journal of Health, Population, and Nutrition. 2001 September;19(3):183–90. - PubMed
-
- Hien BT, Trang do T, Scheutz F, Cam PD, Molbak K, Dalsgaard A. Diarrhoeagenic Escherichia coli and other causes of childhood diarrhoea: a case-control study in children living in a wastewater-use area in Hanoi, Vietnam. Journal of Medical Microbiology. 2007 August;56(Pt 8):1086–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

