Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial
- PMID: 19932356
- PMCID: PMC2914569
- DOI: 10.1016/S0140-6736(09)61492-6
Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial
Abstract
Background: Some studies suggest that patients with asthma who are homozygous for arginine at the 16th amino acid position of the beta2-adrenergic receptor (B16 Arg/Arg) benefit less from treatment with longacting beta2 agonists and inhaled corticosteroids than do those homozygous for glycine (B16 Gly/Gly). We investigated whether there is a genotype-specific response to treatment with a longacting beta2 agonist in combination with inhaled corticosteroid.
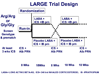
Methods: In this multicentre, randomised, double-blind, placebo-controlled trial, adult patients with moderate asthma were enrolled in pairs matched for forced expiratory volume in 1 s and ethnic origin, according to whether they had the B16 Arg/Arg (n=42) or B16 Gly/Gly (n=45) genotype. Individuals in a matched pair were randomly assigned by computer-generated randomisation sequence to receive inhaled longacting beta2 agonist (salmeterol 50 microg twice a day) or placebo given in a double-blind, crossover design for two 18-week periods. Open-label inhaled corticosteroid (hydrofluoroalkane beclometasone 240 microg twice a day) was given to all participants during the treatment periods. The primary endpoint was morning peak expiratory flow (PEF). Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00200967.
Findings: After 18 weeks of treatment, mean morning PEF in Arg/Arg participants was 21.4 L/min (95% CI 11.8-31.1) higher when participants were assigned to receive salmeterol than when assigned to receive placebo (p<0.0001). In Gly/Gly participants, morning PEF was 21.5 L/min (11.0-32.1) higher when participants were assigned to receive salmeterol than when assigned to receive placebo (p<0.0001). The improvement in PEF did not differ between genotypes (difference [Arg/Arg-Gly/Gly] -0.1, -14.4 to 14.2; p=0.99). In Gly/Gly participants, methacholine PC20 (20% reduction in forced expiratory volume in 1 s; a prespecified secondary outcome) was 2.4 times higher when participants were assigned to salmeterol than when assigned to placebo (p<0.0001). Responsiveness to methacholine did not differ between salmeterol and placebo in Arg/Arg participants (p=0.87). The 2.5 times higher genotype-specific difference in responsiveness to methacholine was significant (1.32 doubling dose difference between genotypes, 0.43-2.21, p=0.0038). Seven Arg/Arg participants (placebo, n=5; salmeterol, n=2) and six Gly/Gly participants (placebo, n=3; salmeterol, n=3) had an asthma exacerbation. Five serious adverse events were reported, one each during the pre-match and run-in phases on open-label inhaled corticosteroid, two during double-blind treatment with salmeterol/inhaled corticosteroid, and one during double-blind treatment with placebo/inhaled corticosteroid. None of the serious events was asthma-related or related to study drugs or procedures.
Interpretation: In asthma patients with B16 Arg/Arg and B16 Gly/Gly genotypes, combination treatment with salmeterol and inhaled corticosteroid improved airway function when compared with inhaled corticosteroid therapy alone. These findings suggest that patients should continue to be treated with longacting beta2 agonists plus moderate-dose inhaled corticosteroids irrespective of B16 genotype. Further investigation is needed to establish the importance of the genotype-specific difference in responsiveness to methacholine.
Funding: National Institutes of Health.
Conflict of interest statement
Dr. Wechsler reports that he has consulted for or participated in advisory boards or speaker bureaus for AstraZeneca, GlaxoSmithKline, Schering Plough, Novartis, Genentech, Merck, Medicinova and Sepracor. Ms. Kunselman reports no conflicts. Dr. Chinchilli reports no conflicts. Dr. Bleecker reports serving as a consultant, giving presentations and performing clinical trials which were administered by Wake Forest University Health Sciences for AstraZeneca, GlaxoSmithKline and Novartis. Dr. Boushey reports research project support from GlaxoSmithKline, participating on a scientific advisory committee for GlaxoSmithKline; ad-hoc consulting for Altana, Boehringer-Ingelheim, Genentech, Nanomix, Novartis, Sumitomo, Theravance and Watermark Research; and honoraria for lectures and presentations from Merck, Novartis, Sanofi-Aventis and Genentech. Dr. Calhoun reports no conflicts. Dr. Ameredes reports no conflicts. Dr. Castro reports no conflicts. Dr. Craig reports performing research with Schering, Merck, GlaxoSmithKline, Boehringer-Ingelheim, Altana, Genentech/Novartis, and Forrest; grants for investigator initiated research from Viropharma, CSL Mehring, GlaxoSmithKline and Merck; honorarium for consultative services from Teva, Alcon, Novartis, Genentech, Dyax, CSL Behring, Viropharma, Shire and Pharming; and honorarium for speaking from Teva Schering, Merck, Astra Zeneca, S. Aventis, Genentech and Novartis. Dr. Denlinger reports no conflicts. Dr. Fahy reports providing consulting services for Abgenix, Aerovance, Amira, Biogen, Cytokinetics, Gilead, Merck, Oxagen and Roche. Dr. Jarjour reports receiving advisory board honorarium and clinical trial support from GlaxoSmithKline; consulting fees from Asthmatx; advisory board compensation, lecture honorarium and clinical trial support from Genentech-Novartis; trial support from MedImmune; and clinical trial support and lecture honorarium from Merck. Dr. Kazani reports no conflicts. Dr. Kim reports no conflicts. Dr. Kraft reports speaker’s bureau for GlaxoSmithKline; advisory board/consultantion for GlaxoSmithKline, Merck, Novartis and Amira; and research funding from GlaxoSmithKline, Bronchus, GE Healthcare, Asthmatx, Novartis and Genentech. Dr. Lazarus reports no conflicts. Dr Lemanske reports receiving speaker honorarium from Merck, Astra Zeneca, Medicus Group, Park Nicolet Institute; consultant honorarium from Astra Zeneca, MAP Pharmaceuticals, Gray Consulting, Smith Research, Merck Childhood Asthma Network, Novartis, Quintiles/ANOVAs and Rchorowitz & Co.; and serving as an author of Up-to-Date. Dr. Markezich reports no conflicts. Dr. Martin reports conflicts with GlaxoSmithKline, Merck, Genentech, Novartis, Altana and Teva. Dr. Permaul reports no conflicts. Dr. Peters reports pharmaceutical trial support as a member of the Wake forest University Clinical Trials Group sponsored by Abaris, Amgen, Altana, Boehringer-Ingelheim, Contocor, Genentech, GlaxoSmithKline, Medimmune, Novartis, Pfizer, Schering and Wyeth; consulting under the auspices of Adelphi, Exocrine, AstraZeneca, Bristol-Meyers Squibb, Ception Therapeutics, Dey, Dyson, Genentech, Johnson & Johnson, Merck, Novartis, RAD Foundation, Respiratory Medicine, Respiratory Research, Sepracor and Teva; and participating in Physician Education Programs sponsored by AdvanceMed, AstraZeneca, Creative Educational Concepts, DIME, Merck Pharmaceuticals, Genentech, Novartis, Practicome, Pri-Med/SCIOS, Respiratory and Allergic Disease (RAD) Foundation, and UpToDate. Dr. Ramsdell reports no conflicts. Dr. Sorkness reports receiving honorarium for Speaker’s bureau and consultation from GlaxoSmithKline; and receiving research support from Pharmaxis and Schering. Dr. Sutherland reports serving as an advisor or consultant to Dey, GlaxoSmithKline and Schering-Plough; and receiving grant funding from Boehringer-Ingelheim, Dey, GlaxoSmithKline, and Novartis. Dr. Szefler reports serving as a consultant for GlaxoSmithKline, Genentech and Merck; and research funding from Ross and GlaxoSmithKline. Dr. Walter reports no conflicts. Dr. Wasserman reports grant support from Merck and Schering-Plough; and serving as a consultant for Amylin. Dr. Israel reports participating in a funded clinical research trial and serving as a consultant with GlaxoSmithKline; participating in a funded clinical research trial with Boehringer Ingelheim; participating in a funded clinical research trial, serving as a consultant, and receiving Speaker’s fees from Novarits; and attending a Speaker’s symposium and serving as a consultant for AstraZeneca.
Figures






Comment in
-
Beta2-receptor polymorphisms in asthma.Lancet. 2009 Nov 21;374(9703):1726-8. doi: 10.1016/S0140-6736(09)61639-1. Lancet. 2009. PMID: 19932345 No abstract available.
-
ADRB2 Arg16Gly polymorphism in the LARGE trial.Lancet. 2010 Feb 27;375(9716):724-5; author reply 725. doi: 10.1016/S0140-6736(10)60303-0. Lancet. 2010. PMID: 20189021 No abstract available.
References
-
- Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997 Nov 13;337(20):1405–1411. - PubMed
-
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006 Jan;129(1):15–26. - PubMed
-
- Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta2-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. 2000. - PubMed
-
- Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004 Oct 23–29;364(9444):1505–1512. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL074227/HL/NHLBI NIH HHS/United States
- U10-HL74218/HL/NHLBI NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- U10-HL074204/HL/NHLBI NIH HHS/United States
- K23 HL004285/HL/NHLBI NIH HHS/United States
- U10-HL74227/HL/NHLBI NIH HHS/United States
- U10-HL74212/HL/NHLBI NIH HHS/United States
- U10 HL074231/HL/NHLBI NIH HHS/United States
- U10 HL074073/HL/NHLBI NIH HHS/United States
- U10 HL074212/HL/NHLBI NIH HHS/United States
- U10-HL74231/HL/NHLBI NIH HHS/United States
- U10 HL074206/HL/NHLBI NIH HHS/United States
- U10-HL74225/HL/NHLBI NIH HHS/United States
- U10 HL074208/HL/NHLBI NIH HHS/United States
- U10 HL074218/HL/NHLBI NIH HHS/United States
- U10-HL074206/HL/NHLBI NIH HHS/United States
- U10 HL074204/HL/NHLBI NIH HHS/United States
- K23-HL04285/HL/NHLBI NIH HHS/United States
- U10 HL074225/HL/NHLBI NIH HHS/United States
- U10-HL074208/HL/NHLBI NIH HHS/United States
- U10-HL74073/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

