Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group
- PMID: 19932542
- PMCID: PMC2804955
- DOI: 10.1053/j.ajkd.2009.08.019
Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group
Abstract
Background: Patients with primary focal segmental glomerulosclerosis (FSGS) resistant to current treatment regimens are at high risk of progression to end-stage kidney disease. Antifibrotic agents, such as tumor necrosis factor alpha antagonists, are a promising strategy to slow or halt the decline in renal function, based on preclinical and clinical data.
Study design: Phase 1 clinical trial to assess the pharmacokinetics, tolerability, and safety of adalimumab, a human monoclonal antibody to tumor necrosis factor alpha.
Setting & participants: 10 patients (4 male and 6 female) aged 16.8 +/- 9.0 years with an estimated glomerular filtration rate of 105 +/- 50 mL/min/1.73 m(2) were studied.
Intervention: Adalimumab, 24 mg/m(2), every 14 days for 16 weeks (total, 9 doses).
Outcomes: Pharmacokinetic assessment, tolerability, and safety.
Measurements: Estimated glomerular filtration rate, proteinuria, and pharmacokinetic assessment after initial dosing and steady state.
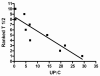
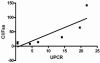
Results: Pharmacokinetic evaluation indicated that the area under the curve was decreased by 54% (P < 0.001) and clearance was increased by 160% (P < 0.01) in patients with resistant FSGS compared with healthy controls and patients with rheumatoid arthritis. Adalimumab was well tolerated with no serious adverse events or infectious complications attributable to the drug. Proteinuria decreased by > or = 50% in 4 of 10 treated patients.
Limitations: Insufficient power to assess the safety or efficacy of adalimumab therapy for patients with resistant FSGS.
Conclusions: Pharmacokinetic assessment showed increased clearance of adalimumab in patients with resistant primary FSGS and validated the need to evaluate the disposition of novel therapies for this disease to define appropriate dosing regimens. The study provides a rationale to evaluate the efficacy of adalimumab as an antifibrotic agent for resistant FSGS in phase 2/3 clinical trials.
Trial registration: ClinicalTrials.gov NCT00814255.
Copyright 2009 National Kidney Foundation, Inc. All rights reserved.
Figures




References
-
- Lopez-Olivo MA, Kallen MA, Ortiz Z, Skidmore B, Suarez-Almazor ME. Quality appraisal of clinical practice guidelines and consensus statements on the use of biologic agents in rheumatoid arthritis: A systemic review. Arthritis Rheum. 2008;59:1625–1638. - PubMed
-
- Shah SB, Hanauer SB. Risks and benefits of the use of concomitant immunosuppressives and biologics in inflammatory bowel disease. Rev Gastroenterol Disord. 2008;8:159–168. - PubMed
-
- Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarasova K, Nemcova D, Mouy R, Sandborg C, Bohnsack J, Elewaut D, Foeldvari I, Gerloni V, Rovensky J, Minden K, Vehe RK, Weiner LW, Horneff G, Huppertz HI, Olson NY, Medich JR, Carcereri-De-Prati R, McIlraith MJ, Giannini EH, Martini A. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–821. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

