A novel composite immunotoxin that suppresses rabies virus production by the infected cells
- PMID: 19932697
- PMCID: PMC2823984
- DOI: 10.1016/j.jim.2009.11.010
A novel composite immunotoxin that suppresses rabies virus production by the infected cells
Abstract
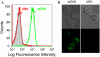
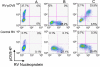
Using strepavidin as a scaffold, we have assembled a composite immunotoxin that consists of recombinant Pseudomonas exotoxin A subunit (PE38) and recombinant 25-D1.16 Fab fragment which recognizes the SIINFEKL (pOV8) peptide from ovalbumin in association with H-2K(b) MHC class I protein. The composite immunotoxin exercises cytotoxicity against H-2K(b+) cells sensitized with pOV8 peptide but not with irrelevant peptide. Specific binding of the immunotoxin to H-2K(b+) cells infected with recombinant rabies virus (RV) expressing pOV8 epitope (RV-pOV8) resulted in the suppression of the production of virus particles by the infected cells. This strategy allows readily produce different immunotoxins with desired specificity by combining various targeting and toxin molecules. The results provide a proof of concept that composite immunotoxins can be utilized as novel immunotherapeutics to stop virus spread in the acute phase of the infection allowing winning time for the development of protective immune response.
2009 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
[Studies of the expression, purification, renaturation and biologic activity of an anti-CEA immunotoxin].Sheng Wu Gong Cheng Xue Bao. 2004 May;20(3):348-51. Sheng Wu Gong Cheng Xue Bao. 2004. PMID: 15971603 Chinese.
-
Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies.Eur J Immunol. 2008 Jun;38(6):1706-20. doi: 10.1002/eji.200737524. Eur J Immunol. 2008. PMID: 18446790 Free PMC article.
-
Expression of VGRNb-PE immunotoxin in transplastomic lettuce (Lactuca sativa L.).Plant Mol Biol. 2018 May;97(1-2):103-112. doi: 10.1007/s11103-018-0726-9. Epub 2018 Apr 10. Plant Mol Biol. 2018. PMID: 29633168
-
Mechanisms of Resistance to Immunotoxins Containing Pseudomonas Exotoxin A in Cancer Therapy.Biomolecules. 2020 Jun 30;10(7):979. doi: 10.3390/biom10070979. Biomolecules. 2020. PMID: 32630017 Free PMC article. Review.
-
A guide to taming a toxin--recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer.FEBS J. 2011 Dec;278(23):4683-700. doi: 10.1111/j.1742-4658.2011.08182.x. Epub 2011 Jun 2. FEBS J. 2011. PMID: 21585657 Free PMC article. Review.
Cited by
-
Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform.PLoS One. 2014 Mar 27;9(3):e93342. doi: 10.1371/journal.pone.0093342. eCollection 2014. PLoS One. 2014. PMID: 24675794 Free PMC article.
-
Antiviral Immunotoxin Against Bovine herpesvirus-1: Targeted Inhibition of Viral Replication and Apoptosis of Infected Cell.Front Microbiol. 2018 Apr 4;9:653. doi: 10.3389/fmicb.2018.00653. eCollection 2018. Front Microbiol. 2018. PMID: 29670605 Free PMC article.
-
Targeting the Inside of Cells with Biologicals: Toxin Routes in a Therapeutic Context.BioDrugs. 2023 Mar;37(2):181-203. doi: 10.1007/s40259-023-00580-y. Epub 2023 Feb 2. BioDrugs. 2023. PMID: 36729328 Free PMC article. Review.
-
Antiviral activity of a single-domain antibody immunotoxin binding to glycoprotein D of herpes simplex virus 2.Antimicrob Agents Chemother. 2015 Jan;59(1):527-35. doi: 10.1128/AAC.03818-14. Epub 2014 Nov 10. Antimicrob Agents Chemother. 2015. PMID: 25385102 Free PMC article.
-
Selective killing of Kaposi's sarcoma-associated herpesvirus lytically infected cells with a recombinant immunotoxin targeting the viral gpK8.1A envelope glycoprotein.MAbs. 2012 Mar-Apr;4(2):233-42. doi: 10.4161/mabs.4.2.19262. Epub 2012 Mar 1. MAbs. 2012. PMID: 22377676 Free PMC article.
References
-
- Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–37. - PubMed
-
- Anikeeva N, Lebedeva T, Krogsgaard M, Tetin SY, Martinez-Hackert E, Kalams SA, Davis MM, Sykulev Y. Distinct molecular mechanisms account for the specificity of two different T-cell receptors. Biochemistry. 2003a;42:4709–4716. - PubMed
-
- Anikeeva N, Lebedeva T, Sumaroka M, Kalams SA, Sykulev Y. Soluble HIV-specific T-cell receptor: expression, purification and analysis of the specificity. J. Immunol. Meth. 2003b;277:75–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

