The synthesis and evaluation of flavone and isoflavone chimeras of novobiocin and derrubone
- PMID: 19932969
- PMCID: PMC2818389
- DOI: 10.1016/j.bmc.2009.10.061
The synthesis and evaluation of flavone and isoflavone chimeras of novobiocin and derrubone
Abstract
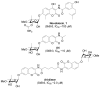
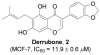
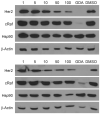
The natural products novobiocin and derrubone have both demonstrated Hsp90 inhibition and structure-activity relationships have been established for each scaffold. Given these compounds share several key structural features, we hypothesized that incorporation of elements from each could provide insight to structural features important for Hsp90 inhibition. Thus, chimeric analogues of novobiocin and derrubone were constructed and evaluated. These studies confirmed that the functionality present at the 3-position of the isoflavone plays a critical role in determining Hsp90 inhibition and suggests that the bicyclic ring system present in both novobiocin and derrubone do not share similar modes of binding.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Figures



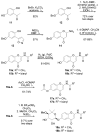



References
-
- Blagosklonny MV. Leukemia. 2002;16:455–462. - PubMed
-
- Workman P. Trends Mol. Med. 2004;10:47–51. - PubMed
-
- Neckers L. Trends Mol. Med. 2002;8:S55–S61. - PubMed
-
- Chiosis G, Vilenchik M, Kim J, Solit D. Drug Disc. Today. 2004;9:881–888. - PubMed
-
- Bishop SC, Burlison JA, Blagg BSJ. Current Cancer Drug Targets. 2007;7:369–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

