Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene
- PMID: 19933191
- PMCID: PMC2846188
- DOI: 10.1167/iovs.09-4569
Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene
Abstract
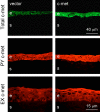
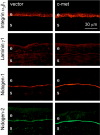
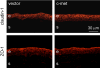
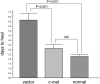
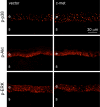
Purpose. Diabetic corneas display altered basement membrane and integrin markers, increased expression of proteinases, decreased hepatocyte growth factor (HGF) receptor, c-met proto-oncogene, and impaired wound healing. Recombinant adenovirus (rAV)-driven c-met overexpression in human organ-cultured corneas was tested for correction of diabetic abnormalities. Methods. Forty-six human corneas obtained postmortem from 23 donors with long-term diabetes (5 with diabetic retinopathy) were organ cultured and transduced with rAV-expressing c-met gene (rAV-cmet) under the cytomegalovirus promoter at approximately 10(8) plaque-forming units per cornea for 48 hours. Each control fellow cornea received control rAV (rAV expressing the beta-galactosidase gene or vector alone). After an additional 4 to 5 days of incubation, 5-mm epithelial wounds were created with n-heptanol, and healing was monitored. The corneas were analyzed afterward by immunohistochemistry and Western blot analysis. Signaling molecule expression and role was examined by immunostaining, phosphokinase antibody arrays, Western blot analysis, and inhibitor analysis. Results. rAV-cmet transduction led to increased epithelial staining for c-met (total, extracellular, and phosphorylated) and normalization of the patterns of select diabetic markers compared with rAV-vector-transduced control fellow corneas. Epithelial wound healing time in c-met-transduced diabetic corneas decreased twofold compared with rAV-vector-transduced corneas and became similar to normal. c-Met action apparently involved increased activation of p38 mitogen-activated protein kinase. c-Met transduction did not change tight junction protein patterns, suggesting unaltered epithelial barrier function. Conclusions. rAV-driven c-met transduction into diabetic corneas appears to restore HGF signaling, normalize diabetic marker patterns, and accelerate wound healing. c-Met gene therapy could be useful for correcting human diabetic corneal abnormalities.
Figures
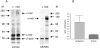

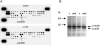

References
-
- Didenko TN, Smoliakova GP, Sorokin EL, Egorov VV. Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes (in Russian). Vestn Oftalmol 1999;115:7–11 - PubMed
-
- Herse PR. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt 1988;65:224–230 - PubMed
-
- Azar DT, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol 1992;110:537–540 - PubMed
-
- Ljubimov AV, Huang Z, Huang GH, et al. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem 1998;46:1033–1041 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

