Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study
- PMID: 19933225
- PMCID: PMC2862178
- DOI: 10.1093/eurheartj/ehp476
Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study
Abstract
Aims: The purpose of this study was to evaluate the Genous(TM) endothelial progenitor cell capturing stent vs. the Taxus Liberté paclitaxel-eluting stent in patients with de novo coronary lesions with a high-risk of coronary restenosis.
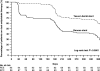
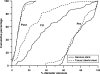
Methods and results: We randomly assigned 193 patients with lesions carrying a high risk of restenosis to have the Genous stent or the Taxus stent implanted. Lesions were considered high risk of restenosis if one of the following applied: chronic total occlusion, lesion length >23 mm, vessel diameter <2.8 mm, or any lesion in a diabetic patient. At 1-year, the rate of the primary end point, target vessel failure (TVF), was 17.3% in the Genous stent group when compared with 10.5% in the Taxus stent group [risk difference (RD) 6.8%, 95% CI -3.1 to 16.7%], a difference predominantly due to a higher incidence of repeat revascularization in patients treated with the Genous stent. In contrast, no stent thrombosis was observed in the Genous stent group compared to 4 stent thromboses in the Taxus stent group (RD -4.2%; 95% CI -10.3 to 0.3%). Repeat angiography between 6 and 12 months in a subgroup of patients showed a significantly higher late loss in the Genous stent compared with the Taxus stent (1.14 +/- 0.64 and 0.55 +/- 0.61 mm).
Conclusion: In patients with lesions carrying a high risk of restenosis, the Genous stent resulted in a non-significant higher rate of TVF compared with the Taxus stent mainly due to more repeat revascularizations in the Genous stent group. There were four stent thromboses with Taxus stent, none with the Genous stent.
Figures

Comment in
-
Endothelial progenitor cell capture stents: will this technology find its niche in contemporary practice?Eur Heart J. 2010 May;31(9):1032-5. doi: 10.1093/eurheartj/ehp591. Epub 2010 Jan 10. Eur Heart J. 2010. PMID: 20064819 No abstract available.
References
-
- Colombo A, Drzewiecki J, Banning A, Grube E, Hauptmann K, Silber S, Dudek D, Fort S, Schiele F, Zmudka K, Guagliumi G, Russell ME. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–794. - PubMed
-
- Grube E, Silber S, Hauptmann KE, Mueller R, Buellesfeld L, Gerckens U, Russell ME. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. - PubMed
-
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban HE, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. - PubMed
-
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. - PubMed
-
- Tsuchida K, Piek JJ, Neuman F, Giesen WJvd, Wiemer M, Zieher AM, Grube E, Haase J, Theusen L, Hamm CW, Veldhof S, Dorange C, Serruys PW. One-year results of a durable polymer everolimus-elutingstent in de novo coronary narrowings (The SPIRIT FIRST Trial) EuroInterv. 2005;1:266–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

