Detection of infective poliovirus by a simple, rapid, and sensitive flow cytometry method based on fluorescence resonance energy transfer technology
- PMID: 19933336
- PMCID: PMC2805220
- DOI: 10.1128/AEM.01851-09
Detection of infective poliovirus by a simple, rapid, and sensitive flow cytometry method based on fluorescence resonance energy transfer technology
Abstract
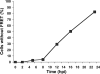
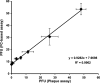
The rapid and effective detection of virus infection is critical for clinical management and prevention of disease spread during an outbreak. Several methods have been developed for this purpose, of which classical serological and viral nucleic acid detection are the most common. We describe an alternative approach that utilizes engineered cells expressing fluorescent proteins undergoing fluorescence resonance energy transfer (FRET) upon cleavage by the viral 2A protease (2A(pro)) as an indication of infection. Quantification of the infectious-virus titers was resolved by using flow cytometry, and utility was demonstrated for the detection of poliovirus 1 (PV1) infection. Engineered buffalo green monkey kidney (BGMK) cells expressing the cyan fluorescent protein (CFP)-yellow fluorescent protein (YFP) substrate linked by a cleavage recognition site for PV1 2A(pro) were infected with different titers of PV1. After incubation at various time points, cells were harvested, washed, and subjected to flow cytometry analysis. The number of infected cells was determined by counting the number of cells with an increased CFP-to-YFP ratio. As early as 5 h postinfection, a significant number of infected cells (3%) was detected by flow cytometry, and cells infected with only 1 PFU were detected after 12 h postinfection. When applied to an environmental water sample spiked with PV1, the flow cytometry-based assay provided a level of sensitivity similar to that of the plaque assay for detecting and quantifying infectious virus particles. This approach, therefore, is more rapid than plaque assays and can be used to detect other viruses that frequently do not form clear plaques on cell cultures.
Figures



References
-
- Bordignon, J., S. C. Pires Ferreira, G. M. Medeiros Caporale, M. L. Carrieri, I. Kotait, H. C. Lima, and C. R. Zanetti. 2002. Flow cytometry assay for intracellular rabies virus detection. J. Virol. Methods 105:181-186. - PubMed
-
- Cromeans, T., M. D. Sobsey, and H. A. Fields. 1987. Development of a plaque assay for a cytopathic, rapidly replicating isolate of hepatitis A virus. J. Med. Virol. 22:45-56. - PubMed
-
- Defoort, J. P., M. Martin, B. Casano, S. Prato, C. Camilla, and V. Fert. 2000. Simultaneous detection of multiplex-amplified human immunodeficiency virus type 1 RNA, hepatitis C virus RNA, and hepatitis B virus DNA using a flow cytometer microsphere-based hybridization assay. J. Clin. Microbiol. 38:1066-1071. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

