Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia
- PMID: 19933342
- PMCID: PMC2805229
- DOI: 10.1128/AEM.01832-09
Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia
Abstract
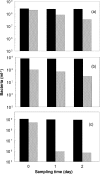
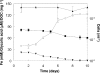
Glycolic acid was detected as an exudate in actively growing cultures of three chemolithotrophic acidophiles that are important in biomining operations, Leptospirillum ferriphilum, Acidithiobacillus (At.) ferrooxidans, and At. caldus. Although similar concentrations of glycolic acid were found in all cases, the concentrations corresponded to ca. 24% of the total dissolved organic carbon (DOC) in cultures of L. ferriphilum but only ca. 5% of the total DOC in cultures of the two Acidithiobacillus spp. Rapid acidification (to pH 1.0) of the culture medium of At. caldus resulted in a large increase in the level of DOC, although the concentration of glycolic acid did not change in proportion. The archaeon Ferroplasma acidiphilum grew in the cell-free spent medium of At. caldus; glycolic acid was not metabolized, although other unidentified compounds in the DOC pool were metabolized. Glycolic acid exhibited levels of toxicity with 21 strains of acidophiles screened similar to those of acetic acid. The most sensitive species were chemolithotrophs (L. ferriphilum and At. ferrivorans), while the most tolerant species were chemoorganotrophs (Acidocella, Acidobacterium, and Ferroplasma species), and the ability to metabolize glycolic acid appeared to be restricted (among acidophiles) to Firmicutes (chiefly Sulfobacillus spp.). Results of this study help explain why Sulfobacillus spp. rather than other acidophiles are the main organic carbon-degrading bacteria in continuously fed stirred tanks used to bioprocess sulfide mineral concentrates and also why temporary cessation of pH control in these systems, resulting in rapid acidification, often results in a plume of the archaeon Ferroplasma.
Figures


References
-
- Aliaga Goltsman, D. S., V. J. Denef, S. W. Singer, N. C. VerBerkmoes, M. Lefsrud, R. S. Mueller, G. J. Dick, C. L. Sun, K. E. Wheeler, A. Zemla, B. J. Baker, L. Hauser, M. Land, M. B. Shah, M. P. Thelen, R. L. Hettich, and J. F. Banfield. 2009. Community genomic and proteomic analysis of chemoautotrophic iron-oxidizing “Leptospirillum rubarum” (group II) and “Leptospirillum ferrodiazotrophum (group III) bacteria in acid mine drainage biofilms. Appl. Environ. Microbiol. 75:4599-4615. - PMC - PubMed
-
- Bridge, T. A. M., and D. B. Johnson. 2000. Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol. J. 17:193-206.
-
- Brierley, J. A., P. R. Norris, D. P. Kelly, and N. W. Le Roux. 1978. Characteristics of a moderately thermophilic and acidophilic iron-oxidizing Thiobacillus. Eur. J. Appl. Microbiol. Biotechnol. 5:291-299.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

