Development of an anatomically detailed MRI-derived rabbit ventricular model and assessment of its impact on simulations of electrophysiological function
- PMID: 19933417
- PMCID: PMC2822578
- DOI: 10.1152/ajpheart.00606.2009
Development of an anatomically detailed MRI-derived rabbit ventricular model and assessment of its impact on simulations of electrophysiological function
Abstract
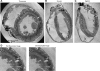
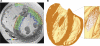
Recent advances in magnetic resonance (MR) imaging technology have unveiled a wealth of information regarding cardiac histoanatomical complexity. However, methods to faithfully translate this level of fine-scale structural detail into computational whole ventricular models are still in their infancy, and, thus, the relevance of this additional complexity for simulations of cardiac function has yet to be elucidated. Here, we describe the development of a highly detailed finite-element computational model (resolution: approximately 125 microm) of rabbit ventricles constructed from high-resolution MR data (raw data resolution: 43 x 43 x 36 microm), including the processes of segmentation (using a combination of level-set approaches), identification of relevant anatomical features, mesh generation, and myocyte orientation representation (using a rule-based approach). Full access is provided to the completed model and MR data. Simulation results were compared with those from a simplified model built from the same images but excluding finer anatomical features (vessels/endocardial structures). Initial simulations showed that the presence of trabeculations can provide shortcut paths for excitation, causing regional differences in activation after pacing between models. Endocardial structures gave rise to small-scale virtual electrodes upon the application of external field stimulation, which appeared to protect parts of the endocardium in the complex model from strong polarizations, whereas intramural virtual electrodes caused by blood vessels and extracellular cleft spaces appeared to reduce polarization of the epicardium. Postshock, these differences resulted in the genesis of new excitation wavefronts that were not observed in more simplified models. Furthermore, global differences in the stimulus recovery rates of apex/base regions were observed, causing differences in the ensuing arrhythmogenic episodes. In conclusion, structurally simplified models are well suited for a large range of cardiac modeling applications. However, important differences are seen when behavior at microscales is relevant, particularly when examining the effects of external electrical stimulation on tissue electrophysiology and arrhythmia induction. This highlights the utility of histoanatomically detailed models for investigations of cardiac function, in particular for future patient-specific modeling.
Figures
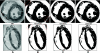






References
-
- Aslanidi OV, Mornev OA, Holden AV. Low-voltage defibrillation in bidomain virtual ventricular tissue: effect of the bath. Comput Cardiol 29: 255–258, 2002
-
- Bishop M, Hales P, Plank G, Gavaghan D, Schneider J, Grau V. Comparison of rule-based and dtmri-derived fiber architecture in a whole rat ventricular computational model. Lecture Notes Comput Sci 5528: 87–96, 2009
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

