A developmental and genetic classification for midbrain-hindbrain malformations
- PMID: 19933510
- PMCID: PMC2792369
- DOI: 10.1093/brain/awp247
A developmental and genetic classification for midbrain-hindbrain malformations
Abstract
Advances in neuroimaging, developmental biology and molecular genetics have increased the understanding of developmental disorders affecting the midbrain and hindbrain, both as isolated anomalies and as part of larger malformation syndromes. However, the understanding of these malformations and their relationships with other malformations, within the central nervous system and in the rest of the body, remains limited. A new classification system is proposed, based wherever possible, upon embryology and genetics. Proposed categories include: (i) malformations secondary to early anteroposterior and dorsoventral patterning defects, or to misspecification of mid-hindbrain germinal zones; (ii) malformations associated with later generalized developmental disorders that significantly affect the brainstem and cerebellum (and have a pathogenesis that is at least partly understood); (iii) localized brain malformations that significantly affect the brain stem and cerebellum (pathogenesis partly or largely understood, includes local proliferation, cell specification, migration and axonal guidance); and (iv) combined hypoplasia and atrophy of putative prenatal onset degenerative disorders. Pertinent embryology is discussed and the classification is justified. This classification will prove useful for both physicians who diagnose and treat patients with these disorders and for clinical scientists who wish to understand better the perturbations of developmental processes that produce them. Importantly, both the classification and its framework remain flexible enough to be easily modified when new embryologic processes are described or new malformations discovered.
Figures

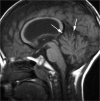
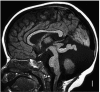
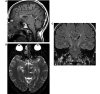
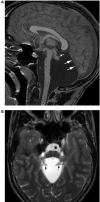
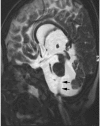
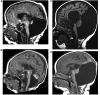
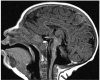
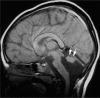
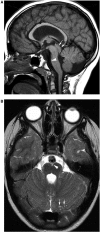
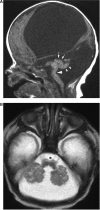
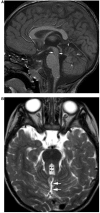
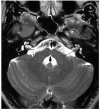
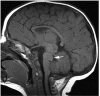
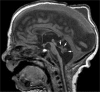
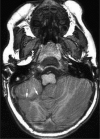
Comment in
-
Sagging and swelling of the midbrain suggest spontaneous intracranial hypotension rather than a malformation.Brain. 2010 Aug;133(Pt 8):e148; author reply e149. doi: 10.1093/brain/awq029. Epub 2010 Mar 7. Brain. 2010. PMID: 20211844 No abstract available.
References
-
- Acosta FL, Jr, Binder DK, Barkovich AJ, Frieden IJ, Gupta N. Neurocutaneous melanosis presenting with hydrocephalus. Case report and review of the literature. J Neurosurg. 2005;102(Suppl 1):96–100. - PubMed
-
- Aicardi J. Aicardi syndrome. Brain Dev. 2005;27:164–71. - PubMed
-
- Aicardi J, Lefebre J, Lerrique-Koechlin A. A new syndrome: spasm in flexion, callosal agenesis, ocular abnormalities. Electroencephalogr Clin Neurophysiol. 1965;19:609–10.
-
- Akasaka-Manya K, Manya H, Endo T. Mutations of the POMT1 gene found in patients with Walker-Warburg syndrome lead to a defect of protein O-mannosylation. Biochem Biophys Res Commun. 2004;325:75–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

