Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women
- PMID: 19933732
- PMCID: PMC2904486
- DOI: 10.1542/peds.2008-2771
Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women
Abstract
Objective: Concern for potential adverse effects of antiretroviral (ARV) chemotherapy used to prevent mother-to-child HIV transmission has led the US Public Health Service to recommend long-term follow-up of ARV-exposed children. Nucleoside reverse transcriptase inhibitor ARV agents can inhibit DNA polymerase gamma, impairing mitochondrial DNA (mtDNA) synthesis and resulting in depletion or dysfunction.
Methods: We measured the mtDNA content of stored peripheral blood mononuclear cells (PBMCs) of 411 healthy children who were born to HIV-uninfected women and 213 uninfected infants who were born to HIV-infected women with or without in utero and neonatal ARV exposure. Cryopreserved PBMC mtDNA was quantified by using the Primagen Retina Mitox assay.
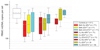
Results: Geometric mean PBMC mtDNA levels were lower at birth in infants who were born to HIV-infected women. Among HIV-exposed children, mtDNA levels were lowest in those who were not exposed to ARVs, higher in those with exposure to zidovudine alone, and higher still in those with combination nucleoside reverse transcriptase inhibitor exposure. A similar pattern was observed in the corresponding women. Levels of mtDNA increased during the first 5 years of life in all HIV-exposed children but achieved normal levels only in those with ARV exposure.
Conclusions: Levels of mtDNA are lower than normal in HIV-exposed children. Contrary to expectation, PBMC mtDNA levels are significantly higher in ARV-exposed, HIV-uninfected infants and their infected mothers compared with ARV-unexposed infants and women. By 5 years, levels of PBMC mtDNA rise to normal concentrations in ARV-exposed children but remain depressed in ARV-unexposed children.
Figures

References
-
- Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995;1(5):417–422. - PubMed
-
- Casula M, Weverling GJ, Wit FW, et al. Mitochondrial DNA and RNA increase in peripheral blood mononuclear cells from HIV-1- infected patients randomized to receive stavudine-containing or stavudine-sparing combination therapy. J Infect Dis. 2005;192(10):1794–1800. - PubMed
-
- Cossarizza A, Pinti M, Moretti L, et al. Mitochondrial functionality and mitochondrial DNA content in lymphocytes of vertically infected human immunodeficiency virus-positive children with highly active antiretroviral therapy-related lipodystrophy. J Infect Dis. 2002;185(3):299–305. - PubMed
-
- de Mendoza C, de Ronde A, Smolders K, et al. Changes in mitochondrial DNA copy number in blood cells from HIV-infected patients undergoing antiretroviral therapy. AIDS Res Hum Retroviruses. 2004;20(3):271–273. - PubMed
-
- López S, Miro O, Martinez E, et al. Mitochondrial effects of antiretroviral therapies in asymptomatic patients. Antivir Ther. 2004;9(1):47–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 RR000188/RR/NCRR NIH HHS/United States
- 9U01 DA 15054/DA/NIDA NIH HHS/United States
- R01 AI94029/AI/NIAID NIH HHS/United States
- R01 HD040777/HD/NICHD NIH HHS/United States
- U01 DA015053/DA/NIDA NIH HHS/United States
- U01 AI034841/AI/NIAID NIH HHS/United States
- U01 AI 50274-01/AI/NIAID NIH HHS/United States
- U01 AI 27541/AI/NIAID NIH HHS/United States
- U01 DA 15053/DA/NIDA NIH HHS/United States
- U01 AI050274/AI/NIAID NIH HHS/United States
- U01 AI034858/AI/NIAID NIH HHS/United States
- U01 DA015054/DA/NIDA NIH HHS/United States
- U01 AI34858/AI/NIAID NIH HHS/United States
- N01 AI085339/AI/NIAID NIH HHS/United States
- M01 RR000645/RR/NCRR NIH HHS/United States
- U01 AI027559/AI/NIAID NIH HHS/United States
- RR00645/RR/NCRR NIH HHS/United States
- N01 HD33162/HD/NICHD NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- M01 RR000865/RR/NCRR NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- U01 HD 41983/HD/NICHD NIH HHS/United States
- U01 AI32921/AI/NIAID NIH HHS/United States
- M01 RR-00188/RR/NCRR NIH HHS/United States
- U01 AI41089/AI/NIAID NIH HHS/United States
- M01 RR-00865/RR/NCRR NIH HHS/United States
- M01 RR-00240/RR/NCRR NIH HHS/United States
- U01 AI027551/AI/NIAID NIH HHS/United States
- U01 AI27559/AI/NIAID NIH HHS/United States
- M01 RR001271/RR/NCRR NIH HHS/United States
- U01 AI 34841/AI/NIAID NIH HHS/United States
- RR00188/RR/NCRR NIH HHS/United States
- U01 AI027550/AI/NIAID NIH HHS/United States
- M01 RR-01271/RR/NCRR NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- N01 AI 85339/AI/NIAID NIH HHS/United States
- U01 AI032907/AI/NIAID NIH HHS/United States
- N01 HD033162/HD/NICHD NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- U01 AI 27550/AI/NIAID NIH HHS/United States
- U01 AI041089/AI/NIAID NIH HHS/United States
- U01 AI027541/AI/NIAID NIH HHS/United States
- HD-3-6117/HD/NICHD NIH HHS/United States
- U01 HD041983/HD/NICHD NIH HHS/United States
- M01 RR000240/RR/NCRR NIH HHS/United States
- U01 AI27551/AI/NIAID NIH HHS/United States
- U01 AI41110/AI/NIAID NIH HHS/United States
- UM1 AI106716/AI/NIAID NIH HHS/United States
- U01 AI32907/AI/NIAID NIH HHS/United States
- P30 AI36211/AI/NIAID NIH HHS/United States
- U01 AI032921/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

