Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial)
- PMID: 19933744
- PMCID: PMC2927615
- DOI: 10.1136/ard.2009.119016
Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial)
Erratum in
- Ann Rheum Dis. 2011 Aug;70(8):1519
Abstract
Background: Several agents provide treatment for established rheumatoid arthritis (RA), but a crucial therapeutic goal is to delay/prevent progression of undifferentiated arthritis (UA) or very early RA.
Objective: To determine the impact of T-cell costimulation modulation in patients with UA or very early RA.
Methods: In this double-blind, phase II, placebocontrolled, 2-year study, anti-cyclic citrullinated peptide (CCP)2-positive patients with UA (not fulfilling the ACR criteria for RA) and clinical synovitis of two or more joints were randomised to abatacept ( approximately 10 mg/kg) or placebo for 6 months; the study drug was then terminated. The primary end point was development of RA (by ACR criteria) at year 1. Patients were monitored by radiography, MRI, CCP2, rheumatoid factor and 28 joint count Disease Activity Score (DAS28) over 2 years.
Results: At year 1, 12/26 (46%) abatacept-treated versus 16/24 (67%) placebo-treated patients developed RA (difference (95% CI) -20.5% (-47.4% to 7.8%)). Adjusted mean changes from baseline to year 1 in Genant-modified Sharp radiographic scores for abatacepttreated versus placebo-treated patients, respectively, were 0 versus 1.1 for total score, and 0 versus 0.9 for erosion score. Mean changes from baseline to year 1 in MRI erosion, osteitis and synovitis scores were 0, 0.2 and 0.2, respectively, versus 5.0, 6.7 and 2.3 in the abatacept versus placebo groups. Safety was comparable between groups; serious adverse events occurred in one patient (3.6%) in each group.
Conclusion: Abatacept delayed progression of UA/very early RA in some patients. An impact on radiographic and MRI inhibition was seen, which was maintained for 6 months after treatment stopped. This suggests that it is possible to alter the progression of RA by modulating T-cell responses at a very early stage of disease. Trial registration number NCT00124449.
Conflict of interest statement
Figures

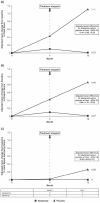
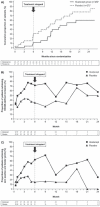
Comment in
-
When does rheumatoid arthritis start and can it be stopped before it does?Ann Rheum Dis. 2010 Mar;69(3):473-5. doi: 10.1136/ard.2009.116020. Epub 2010 Mar 9. Ann Rheum Dis. 2010. PMID: 20215139 No abstract available.
References
-
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2008;58(Suppl):S126–35 - PubMed
-
- Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82 - PubMed
-
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9 - PubMed
-
- van der Helm-van Mil AH, Verpoort KN, Breedveld FC, et al. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum 2006;54:1117–21 - PubMed
-
- Huizinga TW, Amos CI, van der Helm-van Mil AH, et al. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum 2005;52:3433–8 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

