The gating isomerization of neuromuscular acetylcholine receptors
- PMID: 19933754
- PMCID: PMC2828132
- DOI: 10.1113/jphysiol.2009.182774
The gating isomerization of neuromuscular acetylcholine receptors
Abstract
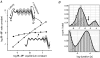
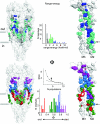
Acetylcholine receptor-channels are allosteric proteins that isomerize ('gate') between conformations that have a low vs. high affinity for the transmitter and conductance for ions. In order to comprehend the mechanism by which the affinity and conductance changes are linked it is of value to know the magnitude, timing and distribution of energy flowing through the system. Knowing both the di- and unliganded gating equilibrium constants (E(2) and E(0)) is a foundation for understanding the AChR gating mechanism and for engineering both the ligand and the protein to operate in predictable ways. In adult mouse neuromuscular receptors activated by acetylcholine, E(2) = 28 and E(0) approximately 6.5 x 10(7). At each (equivalent) transmitter binding site acetylcholine provides approximately 5.2 kcal mol(1) to motivate the isomerization. The partial agonist choline provides approximately 3.3 kcal mol(1). The relative time of a residue's gating energy change is revealed by the slope of its rate-equilibrium constant relationship. A map of this parameter suggests that energy propagates as a conformational cascade between the transmitter binding sites and the gate region. Although gating energy changes are widespread throughout the protein, some residues are particularly sensitive to perturbations. Several specific proposals for the structural events that comprise the gating conformational cascade are discussed.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

