The Enhancing Secondary Prevention in Coronary Artery Disease trial
- PMID: 19933787
- PMCID: PMC2789127
- DOI: 10.1503/cmaj.090917
The Enhancing Secondary Prevention in Coronary Artery Disease trial
Abstract
Background: Proven efficacious therapies are sometimes underused in patients with chronic cardiac conditions, resulting in suboptimal outcomes. We evaluated whether evidence summaries, which were either unsigned or signed by local opinion leaders, improved the quality of secondary prevention care delivered by primary care physicians of patients with coronary artery disease.
Methods: We performed a randomized trial, clustered at the level of the primary care physician, with 3 study arms: control, unsigned statements or opinion leader statements. The statements were faxed to primary care physicians of adults with coronary artery disease at the time of elective cardiac catheterization. The primary outcome was improvement in statin management (initiation or dose increase) 6 months after catheterization.
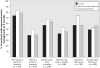
Results: We enrolled 480 adults from 252 practices. Although statin use was high at baseline (n=316 [66%]), most patients were taking a low dose (mean 32% of the guideline-recommended dose), and their low-density lipoprotein (LDL) cholesterol levels were elevated (mean 3.09 mmol/L). Six months after catheterization, statin management had improved in 79 of 157 patients (50%) in the control arm, 85 of 158 (54%) patients in the unsigned statement group (adjusted odds ratio [OR] 1.18, 95% CI 0.71-1.94, p=0.52) and 99 of 165 (60%) patients in the opinion leader statement group (adjusted OR 1.51, 95% CI 0.94-2.42, p=0.09). The mean fasting LDL cholesterol levels after 6 months were similar in all 3 study arms: 2.35 (standard deviation [SD] 0.86) mmol/L in the control arm compared with 2.24 (SD 0.73) among those in the opinion leader group (p=0.48) and 2.19 (SD 0.68) in the unsigned statement group (p=0.32).
Interpretation: Faxed evidence reminders for primary care physicians, even when endorsed by local opinion leaders, were insufficient to optimize the quality of care for adults with coronary artery disease. ClinicalTrials.gov trial register no. NCT00175240.
Figures
Comment in
-
Why didn't it work?CMAJ. 2010 Feb 9;182(2):175. doi: 10.1503/cmaj.110-2020. CMAJ. 2010. PMID: 20142387 Free PMC article. No abstract available.
References
-
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–9. - PubMed
-
- EUROASPIRE I and II Group. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. Lancet. 2001;357:995–1001. - PubMed
-
- Pilote L, Beck CA, Karp I, et al. Secondary prevention after acute myocardial infarction in four Canadian provinces, 1997–2000. Can J Cardiol. 2004;2:61–7. - PubMed
-
- Newby LK, Allen LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
