Voriconazole pharmacokinetics in liver transplant recipients
- PMID: 19933807
- PMCID: PMC2812143
- DOI: 10.1128/AAC.00429-09
Voriconazole pharmacokinetics in liver transplant recipients
Abstract
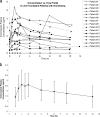
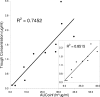
The objective of this study was to evaluate the pharmacokinetics of voriconazole and the potential correlations between pharmacokinetic parameters and patient variables in liver transplant patients on a fixed-dose prophylactic regimen. Multiple blood samples were collected within one dosing interval from 15 patients who were initiated on a prophylactic regimen of voriconazole at 200 mg enterally (tablets) twice daily starting immediately posttransplant. Voriconazole plasma concentrations were measured using high-pressure liquid chromatography (HPLC). Noncompartmental pharmacokinetic analysis was performed to estimate pharmacokinetic parameters. The mean apparent systemic clearance over bioavailability (CL/F), apparent steady-state volume of distribution over bioavailability (Vss/F), and half-life (t1/2) were 5.8+/-5.5 liters/h, 94.5+/-54.9 liters, and 15.7+/-7.0 h, respectively. There was a good correlation between the area under the concentration-time curve from 0 h to infinity (AUC0-infinity) and trough voriconazole plasma concentrations. t1/2, maximum drug concentration in plasma (Cmax), trough level, AUC0-infinity, area under the first moment of the concentration-time curve from 0 h to infinity (AUMC0-infinity), and mean residence time from 0 h to infinity (MRT0-infinity) were significantly correlated with postoperative time. t1/2, lambda, AUC0-infinity, and CL/F were significantly correlated with indices of liver function (aspartate transaminase [AST], total bilirubin, and international normalized ratio [INR]). The Cmax, last concentration in plasma at 12 h (Clast), AUMC0-infinity, and MRT0-infinity were significantly lower in the presence of deficient CYP2C19*2 alleles. Donor characteristics had no significant correlation with any of the pharmacokinetic parameters estimated. A fixed dosing regimen of voriconazole results in a highly variable exposure of voriconazole in liver transplant patients. Given that trough voriconazole concentration is a good measure of drug exposure (AUC), the voriconazole dose can be individualized based on trough concentration measurements in liver transplant patients.
Figures

References
-
- Boyd, A. E., S. Modi, et al. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241-1244. - PubMed
-
- Capitano, B., B. Potoski, et al. 2004. Pharmacokinetics of voriconazole in lung transplant patients, abstr. A-39. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
-
- Denning, D. W., P. Ribaud, et al. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. - PubMed
-
- Desta, Z., X. Zhao, et al. 2002. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 41:913-958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

