Plasmodium falciparum merozoite surface protein 1 (MSP-1)-MSP-3 chimeric protein: immunogenicity determined with human-compatible adjuvants and induction of protective immune response
- PMID: 19933832
- PMCID: PMC2812216
- DOI: 10.1128/IAI.00427-09
Plasmodium falciparum merozoite surface protein 1 (MSP-1)-MSP-3 chimeric protein: immunogenicity determined with human-compatible adjuvants and induction of protective immune response
Abstract
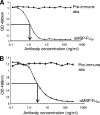
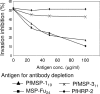
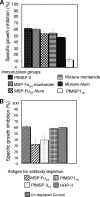
A chimeric gene, MSP-Fu(24), was constructed by genetically coupling immunodominant, conserved regions of the two leading malaria vaccine candidates, Plasmodium falciparum merozoite surface protein 1 (C-terminal 19-kDa region [PfMSP-1(19)]) and merozoite surface protein 3 (11-kDa conserved region [PfMSP-3(11)]). The recombinant MSP-Fu(24) protein was produced in Escherichia coli cells and purified to homogeneity by a two-step purification process with a yield of approximately 30 mg/liter. Analyses of conformational properties of MSP-Fu(24) using PfMSP-1(19)-specific monoclonal antibody showed that the conformational epitopes of PfMSP-1(19) that may be critical for the generation of the antiparasitic immune response remained intact in the fusion protein. Recombinant MSP-Fu(24) was highly immunogenic in mice and in rabbits when formulated with two different human-compatible adjuvants and induced an immune response against both PfMSP-1(19) and PfMSP-3(11). Purified anti-MSP-Fu(24) antibodies showed invasion inhibition of P. falciparum 3D7 and FCR parasites, and this effect was found to be dependent on antibodies specific for the PfMSP-1(19) component. The protective potential of MSP-Fu(24) was demonstrated by in vitro parasite growth inhibition using an antibody-dependent cell inhibition (ADCI) assay with anti-MSP-Fu(24) antibodies. Overall, the antiparasitic activity was mediated by a combination of growth-inhibitory antibodies generated by both the PfMSP-1(19) and PfMSP-3(11) components of the MSP-Fu(24) protein. The antiparasitic activities elicited by anti-MSP-Fu(24) antibodies were comparable to those elicited by antibodies generated with immunization with a physical mixture of two component antigens, PfMSP-1(19) and PfMSP-3(11). The fusion protein induces a protective immune response with human-compatible adjuvants and may form a part of a multicomponent malaria vaccine.
Figures


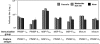

References
-
- Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. - PMC - PubMed
-
- Burghaus, P. A., and A. A. Holder. 1994. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol. Biochem. Parasitol. 64:165-169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

