RasGRP1 is required for human NK cell function
- PMID: 19933860
- PMCID: PMC2896689
- DOI: 10.4049/jimmunol.0902012
RasGRP1 is required for human NK cell function
Abstract
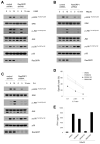
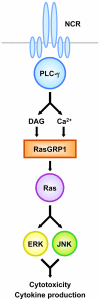
Cross-linking of NK activating receptors activates phospholipase-gamma and subsequently induces diacylglycerol and Ca(2+) as second messengers of signal transduction. Previous studies reported that Ras guanyl nucleotide-releasing protein (RasGRP) 1, which is activated by diacylglycerol and Ca(2+), is crucial for TCR-mediated Ras-ERK activation. We now report that RasGRP1, which can also be detected in human NK cells, plays an essential role in NK cell effector functions. To examine the role of RasGRP1 in NK cell functions, the expression of RasGRP1 was suppressed using RNA interference. Knockdown of RasGRP1 significantly blocked ITAM-dependent cytokine production as well as NK cytotoxicity. Biochemically, RasGRP1-knockdown NK cells showed markedly decreased ability to activate Ras, ERK, and JNK. Activation of the Ras-MAPK pathway was independently shown to be indispensable for NK cell effector functions via the use of specific pharmacological inhibitors. Our results reveal that RasGRP1 is required for the activation of the Ras-MAPK pathway leading to NK cell effector functions. Moreover, our data suggest that RasGRP1 might act as an important bridge between phospholipase-gamma activation and NK cell effector functions via the Ras-MAPK pathway.
Figures



Similar articles
-
A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells.Mol Cell Biol. 2005 Jun;25(11):4426-41. doi: 10.1128/MCB.25.11.4426-4441.2005. Mol Cell Biol. 2005. PMID: 15899849 Free PMC article.
-
An essential role for RasGRP1 in mast cell function and IgE-mediated allergic response.J Exp Med. 2007 Jan 22;204(1):93-103. doi: 10.1084/jem.20061598. Epub 2006 Dec 26. J Exp Med. 2007. PMID: 17190838 Free PMC article.
-
RASGRP1 deficiency causes immunodeficiency with impaired cytoskeletal dynamics.Nat Immunol. 2016 Dec;17(12):1352-1360. doi: 10.1038/ni.3575. Epub 2016 Oct 24. Nat Immunol. 2016. PMID: 27776107 Free PMC article.
-
A Focused Review of Ras Guanine Nucleotide-Releasing Protein 1 in Immune Cells and Cancer.Int J Mol Sci. 2023 Jan 13;24(2):1652. doi: 10.3390/ijms24021652. Int J Mol Sci. 2023. PMID: 36675167 Free PMC article. Review.
-
Exploring the multifaceted role of RASGRP1 in disease: immune, neural, metabolic, and oncogenic perspectives.Cell Cycle. 2024 Mar;23(6):722-746. doi: 10.1080/15384101.2024.2366009. Epub 2024 Jun 12. Cell Cycle. 2024. PMID: 38865342 Free PMC article. Review.
Cited by
-
RasGRP1 promotes the acute inflammatory response and restricts inflammation-associated cancer cell growth.Nat Commun. 2022 Nov 16;13(1):7001. doi: 10.1038/s41467-022-34659-x. Nat Commun. 2022. PMID: 36385095 Free PMC article.
-
Farnesyltransferase inhibitor tipifarnib inhibits Rheb prenylation and stabilizes Bax in acute myelogenous leukemia cells.Haematologica. 2014 Jan;99(1):60-9. doi: 10.3324/haematol.2013.087734. Epub 2013 Aug 30. Haematologica. 2014. PMID: 23996484 Free PMC article.
-
IGF-1 promotes the development and cytotoxic activity of human NK cells.Nat Commun. 2013;4:1479. doi: 10.1038/ncomms2484. Nat Commun. 2013. PMID: 23403580 Free PMC article.
-
In silico search for modifier genes associated with pancreatic and liver disease in Cystic Fibrosis.PLoS One. 2017 Mar 24;12(3):e0173822. doi: 10.1371/journal.pone.0173822. eCollection 2017. PLoS One. 2017. PMID: 28339466 Free PMC article.
-
Novel Mutations in RASGRP1 are Associated with Immunodeficiency, Immune Dysregulation, and EBV-Induced Lymphoma.J Clin Immunol. 2018 Aug;38(6):699-710. doi: 10.1007/s10875-018-0533-8. Epub 2018 Jul 20. J Clin Immunol. 2018. PMID: 30030704
References
-
- Perussia B. The Cytokine Profile of Resting and Activated NK Cells. Methods. 1996;9:370–378. - PubMed
-
- Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–316. - PubMed
-
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. - PubMed
-
- Tassi I, Klesney-Tait J, Colonna M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol Rev. 2006;214:92–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

