The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response
- PMID: 19933869
- PMCID: PMC3374958
- DOI: 10.4049/jimmunol.0803903
The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response
Abstract
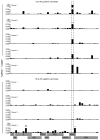
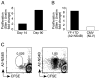
The live yellow fever vaccine (YF-17D) offers a unique opportunity to study memory CD8(+) T cell differentiation in humans following an acute viral infection. We have performed a comprehensive analysis of the virus-specific CD8(+) T cell response using overlapping peptides spanning the entire viral genome. Our results showed that the YF-17D vaccine induces a broad CD8(+) T cell response targeting several epitopes within each viral protein. We identified a dominant HLA-A2-restricted epitope in the NS4B protein and used tetramers specific for this epitope to track the CD8(+) T cell response over a 2 year period. This longitudinal analysis showed the following. 1) Memory CD8(+) T cells appear to pass through an effector phase and then gradually down-regulate expression of activation markers and effector molecules. 2) This effector phase was characterized by down-regulation of CD127, Bcl-2, CCR7, and CD45RA and was followed by a substantial contraction resulting in a pool of memory T cells that re-expressed CD127, Bcl-2, and CD45RA. 3) These memory cells were polyfunctional in terms of degranulation and production of the cytokines IFN-gamma, TNF-alpha, IL-2, and MIP-1beta. 4) The YF-17D-specific memory CD8(+) T cells had a phenotype (CCR7(-)CD45RA(+)) that is typically associated with terminally differentiated cells with limited proliferative capacity (T(EMRA)). However, these cells exhibited robust proliferative potential showing that expression of CD45RA may not always associate with terminal differentiation and, in fact, may be an indicator of highly functional memory CD8(+) T cells generated after acute viral infections.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures






References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. - PubMed
-
- Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. - PubMed
-
- Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

