Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial
- PMID: 19933912
- PMCID: PMC4295034
- DOI: 10.1200/JCO.2009.24.2669
Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial
Abstract
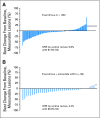
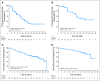
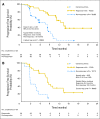
PURPOSE No established treatment exists for pancreatic neuroendocrine tumor (NET) progression after failure of chemotherapy. Everolimus (RAD001), an oral inhibitor of mammalian target of rapamycin, in combination with octreotide has demonstrated encouraging antitumor activity in patients with NETs. PATIENTS AND METHODS This open-label, phase II study assessed the clinical activity of everolimus in patients with metastatic pancreatic NETs who experienced progression on or after chemotherapy. Patients were stratified by prior octreotide therapy (stratum 1: everolimus 10 mg/d, n = 115; stratum 2: everolimus 10 mg/d plus octreotide long-acting release [LAR], n = 45). Tumor assessments (using Response Evaluation Criteria in Solid Tumors) were performed every 3 months. Chromogranin A (CgA) and neuron-specific enolase (NSE) were assessed monthly if elevated at baseline. Trough concentrations of everolimus and octreotide were assessed. Results By central radiology review, in stratum 1, there were 11 partial responses (9.6%), 78 patients (67.8%) with stable disease (SD), and 16 patients (13.9%) with progressive disease; median progression-free survival (PFS) was 9.7 months. In stratum 2, there were two partial responses (4.4%), 36 patients (80%) with SD, and no patients with progressive disease; median PFS was 16.7 months. Patients with an early CgA or NSE response had a longer PFS compared with patients without an early response. Coadministration of octreotide LAR and everolimus did not impact exposure to either drug. Most adverse events were mild to moderate and were consistent with those previously seen with everolimus. CONCLUSION Daily everolimus, with or without concomitant octreotide LAR, demonstrates antitumor activity as measured by objective response rate and PFS and is well tolerated in patients with advanced pancreatic NETs after failure of prior systemic chemotherapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. - PubMed
-
- Lam KY, Lo CY. Pancreatic endocrine tumour: A 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol. 1997;23:36–42. - PubMed
-
- Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: Survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078–1085. - PubMed
-
- Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

