Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review
- PMID: 19933921
- PMCID: PMC2799236
- DOI: 10.1200/JCO.2008.19.9844
Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review
Abstract
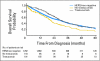
PURPOSE The purpose of this study was to determine whether trastuzumab improves prognosis of women with metastatic human epidermal growth factor receptor 2 (HER2)/neu -positive breast cancer beyond that of women with HER2/neu-negative disease. PATIENTS AND METHODS Two thousand ninety-one women with metastatic breast cancer diagnosed from 1991 to 2007, with known HER2/neu status and who had not received trastuzumab in the adjuvant setting, were identified. Disease was classified into the following three groups: HER2/neu negative, HER2/neu positive without first-line trastuzumab treatment, and HER2/neu positive with first-line trastuzumab treatment. Overall survival (OS) was estimated using the Kaplan-Meier product-limit method and compared between groups with the log-rank test. Cox proportional hazards models were used to determine associations between OS and HER2/neu status after controlling for patient characteristics. Results One hundred eighteen patients (5.6%) had HER2/neu-positive disease without trastuzumab treatment, 191 (9.1%) had HER2/neu-positive disease and received trastuzumab treatment, and 1,782 (85.3%) had HER2/neu-negative disease. Median-follow-up was 16.9 months. One-year survival rates among patients with HER2/neu-negative disease, HER2/neu-positive disease and trastuzumab treatment, and HER2/neu-positive disease and no trastuzumab treatment were 75.1% (95% CI, 72.9% to 77.2%), 86.6% (95% CI, 80.8% to 90.8%), and 70.2% (95% CI, 60.3% to 78.1%), respectively. In a multivariable model, women with HER2/neu-positive disease who received trastuzumab had a 44% reduction in the risk of death compared with women with HER2/neu-negative disease (hazard ratio [HR] = 0.56; 95% CI, 0.45 to 0.69; P < .0001). This HR varied with time and was significant for the first 24 months and not significant after 24 months. CONCLUSION Our results show that women with HER2/neu-positive disease who received trastuzumab had improved prognosis compared with women with HER2/neu-negative disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Improved prognosis by trastuzumab of women with HER2-positive breast cancer compared with those with HER2-negative disease.J Clin Oncol. 2010 Jul 10;28(20):e337; author reply e338-9. doi: 10.1200/JCO.2010.28.2525. Epub 2010 May 17. J Clin Oncol. 2010. PMID: 20479395 No abstract available.
References
-
- Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230:1132–1139. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Seshadri R, Firgaira FA, Horsfall DJ, et al. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer: The South Australian Breast Cancer Study Group. J Clin Oncol. 1993;11:1936–1942. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous