Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions
- PMID: 19933931
- PMCID: PMC2825656
- DOI: 10.1161/CIRCULATIONAHA.109.879429
Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions
Abstract
Background: MicroRNAs (miRNAs/miRs) are small conserved RNA molecules of 22 nucleotides that negatively modulate gene expression primarily through base paring to the 3' untranslated region of target messenger RNAs. The muscle-specific miR-1 has been implicated in cardiac hypertrophy, heart development, cardiac stem cell differentiation, and arrhythmias through targeting of regulatory proteins. In this study, we investigated the molecular mechanisms through which miR-1 intervenes in regulation of muscle cell growth and differentiation.
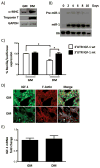
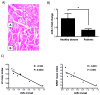
Methods and results: On the basis of bioinformatics tools, biochemical assays, and in vivo models, we demonstrate that (1) insulin-like growth factor-1 (IGF-1) and IGF-1 receptor are targets of miR-1; (2) miR-1 and IGF-1 protein levels are correlated inversely in models of cardiac hypertrophy and failure as well as in the C2C12 skeletal muscle cell model of differentiation; (3) the activation state of the IGF-1 signal transduction cascade reciprocally regulates miR-1 expression through the Foxo3a transcription factor; and (4) miR-1 expression correlates inversely with cardiac mass and thickness in myocardial biopsies of acromegalic patients, in which IGF-1 is overproduced after aberrant synthesis of growth hormone.
Conclusions: Our results reveal a critical role of miR-1 in mediating the effects of the IGF-1 pathway and demonstrate a feedback loop between miR-1 expression and the IGF-1 signal transduction cascade.
Figures





References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, Valtieri M, Calin GA, Liu CG, Sorrentino A, Croce CM, Peschle C. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci U S A. 2005;102:18081–18086. - PMC - PubMed
-
- Xu P, Guo M, Hay BA. MicroRNAs and the regulation of cell death. Trends Genet. 2004;20:617–624. - PubMed
-
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

