Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake
- PMID: 19933997
- PMCID: PMC2809973
- DOI: 10.2337/db09-1198
Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake
Abstract
Objective: Prokineticin 2 (PK2) is a hypothalamic neuropeptide expressed in central nervous system areas known to be involved in food intake. We therefore hypothesized that PK2 plays a role in energy homeostasis.
Research design and methods: We investigated the effect of nutritional status on hypothalamic PK2 expression and effects of PK2 on the regulation of food intake by intracerebroventricular (ICV) injection of PK2 and anti-PK2 antibody. Subsequently, we investigated the potential mechanism of action by determining sites of neuronal activation after ICV injection of PK2, the hypothalamic site of action of PK2, and interaction between PK2 and other hypothalamic neuropeptides regulating energy homeostasis. To investigate PK2's potential as a therapeutic target, we investigated the effect of chronic administration in lean and obese mice.
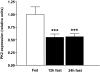
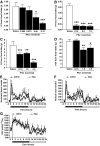
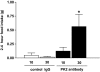
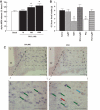
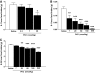
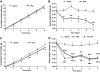
Results: Hypothalamic PK2 expression was reduced by fasting. ICV administration of PK2 to rats potently inhibited food intake, whereas anti-PK2 antibody increased food intake, suggesting that PK2 is an anorectic neuropeptide. ICV administration of PK2 increased c-fos expression in proopiomelanocortin neurons of the arcuate nucleus (ARC) of the hypothalamus. In keeping with this, PK2 administration into the ARC reduced food intake and PK2 increased the release of alpha-melanocyte-stimulating hormone (alpha-MSH) from ex vivo hypothalamic explants. In addition, ICV coadministration of the alpha-MSH antagonist agouti-related peptide blocked the anorexigenic effects of PK2. Chronic peripheral administration of PK2 reduced food and body weight in lean and obese mice.
Conclusions: This is the first report showing that PK2 has a role in appetite regulation and its anorectic effect is mediated partly via the melanocortin system.
Figures

References
-
- Wechselberger C, Puglisi R, Engel E, Lepperdinger G, Boitani C, Kreil G: The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett 1999;462:177–181 - PubMed
-
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY: Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol 2001;59:692–698 - PubMed
-
- LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N: Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 2001;412:877–884 - PubMed
-
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY: Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem 2002;277:19276–19280 - PubMed
-
- Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M: Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun 2002;293:396–402 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- G0700495/MRC_/Medical Research Council/United Kingdom
- BB/D525064/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F021704/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E021972/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

