Human recombinant ACE2 reduces the progression of diabetic nephropathy
- PMID: 19934006
- PMCID: PMC2809962
- DOI: 10.2337/db09-1218
Human recombinant ACE2 reduces the progression of diabetic nephropathy
Erratum in
- Diabetes. 2010 Apr;59(4):1113-4
Abstract
Objective: Diabetic nephropathy is one of the most common causes of end-stage renal failure. Inhibition of ACE2 function accelerates diabetic kidney injury, whereas renal ACE2 is downregulated in diabetic nephropathy. We examined the ability of human recombinant ACE2 (hrACE2) to slow the progression of diabetic kidney injury.
Research design and methods: Male 12-week-old diabetic Akita mice (Ins2(WT/C96Y)) and control C57BL/6J mice (Ins2(WT/WT)) were injected daily with placebo or with rhACE2 (2 mg/kg, i.p.) for 4 weeks. Albumin excretion, gene expression, histomorphometry, NADPH oxidase activity, and peptide levels were examined. The effect of hrACE2 on high glucose and angiotensin II (ANG II)-induced changes was also examined in cultured mesangial cells.
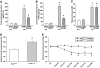
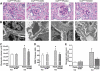
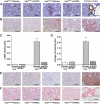
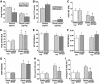
Results: Treatment with hrACE2 increased plasma ACE2 activity, normalized blood pressure, and reduced the urinary albumin excretion in Akita Ins2(WT/C96Y) mice in association with a decreased glomerular mesangial matrix expansion and normalization of increased alpha-smooth muscle actin and collagen III expression. Human recombinant ACE2 increased ANG 1-7 levels, lowered ANG II levels, and reduced NADPH oxidase activity. mRNA levels for p47(phox) and NOX2 and protein levels for protein kinase Calpha (PKCalpha) and PKCbeta1 were also normalized by treatment with hrACE2. In vitro, hrACE2 attenuated both high glucose and ANG II-induced oxidative stress and NADPH oxidase activity.
Conclusions: Treatment with hrACE2 attenuates diabetic kidney injury in the Akita mouse in association with a reduction in blood pressure and a decrease in NADPH oxidase activity. In vitro studies show that the protective effect of hrACE2 is due to reduction in ANG II and an increase in ANG 1-7 signaling.
Figures


References
-
- Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007;72:247–259 - PubMed
-
- Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ: Chronic kidney disease: common, harmful, and treatable—World Kidney Day 2007. J Am Soc Nephrol 2007;18:374–378 - PubMed
-
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–2047 - PubMed
-
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy: the Collaborative Study Group. N Engl J Med 1993;329:1456–1462 - PubMed
-
- Cooper ME: Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 1998;352:213–219 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

