Engineered zinc finger protein-mediated VEGF-a activation restores deficient VEGF-a in sensory neurons in experimental diabetes
- PMID: 19934008
- PMCID: PMC2809974
- DOI: 10.2337/db08-1526
Engineered zinc finger protein-mediated VEGF-a activation restores deficient VEGF-a in sensory neurons in experimental diabetes
Abstract
Objective: The objectives of the study were to evaluate retrograde axonal transport of vascular endothelial growth factor A (VEGF-A) protein to sensory neurons after intramuscular administration of an engineered zinc finger protein activator of endogenous VEGF-A (VZ+434) in an experimental model of diabetes, and to characterize the VEGF-A target neurons.
Research design and methods: We compared the expression of VEGF-A in lumbar (L)4/5 dorsal root ganglia (DRG) of control rats and VZ+434-treated and untreated streptozotocin (STZ)-induced diabetic rats. In addition, axonal transport of VEGF-A, activation of signal transduction pathways in the DRG, and mechanical sensitivity were assessed.
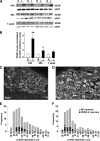
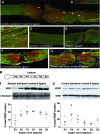
Results: VEGF-A immunoreactivity (IR) was detected in small- to medium-diameter neurons in DRG of control rats. Fewer VEGF-A-IR neurons were observed in DRG from STZ-induced diabetic rats; this decrease was confirmed and quantified by Western blotting. VZ+434 administration resulted in a significant increase in VEGF-A protein expression in ipsilateral DRG, 24 h after injection. VEGF-A was axonally transported to the DRG via the sciatic nerve. VZ+434 administration resulted in significant activation of AKT in the ipsilateral DRG by 48 h that was sustained for 1 week after injection. VZ+434 protected against mechanical allodynia 8 weeks after STZ injection.
Conclusions: Intramuscular administration of VZ+434 increases VEGF-A protein levels in L4/5 DRG, correcting the deficit observed after induction of diabetes, and protects against mechanical allodynia. Elevated VEGF-A levels result from retrograde axonal transport and are associated with altered signal transduction, via the phosphatidylinositol 3'-kinase pathway. These data support a neuroprotective role for VEGF-A in the therapeutic actions of VZ+434 and suggest a mechanism by which VEGF-A exerts this activity.
Figures





Similar articles
-
Diabetes and axotomy-induced deficits in retrograde axonal transport of nerve growth factor correlate with decreased levels of p75LNTR protein in lumbar dorsal root ganglia.Brain Res Mol Brain Res. 1997 Nov;51(1-2):82-90. doi: 10.1016/s0169-328x(97)00215-5. Brain Res Mol Brain Res. 1997. PMID: 9427509
-
Vascular endothelial growth factor expression in peripheral nerves and dorsal root ganglia in diabetic neuropathy in rats.Neurosci Lett. 1999 Mar 12;262(3):159-62. doi: 10.1016/s0304-3940(99)00064-6. Neurosci Lett. 1999. PMID: 10218880
-
RNA-Binding Proteins HuB, HuC, and HuD are Distinctly Regulated in Dorsal Root Ganglia Neurons from STZ-Sensitive Compared to STZ-Resistant Diabetic Mice.Int J Mol Sci. 2019 Apr 22;20(8):1965. doi: 10.3390/ijms20081965. Int J Mol Sci. 2019. PMID: 31013625 Free PMC article.
-
Tumor necrosis factor-α elevates neurite outgrowth through an NF-κB-dependent pathway in cultured adult sensory neurons: Diminished expression in diabetes may contribute to sensory neuropathy.Brain Res. 2011 Nov 14;1423:87-95. doi: 10.1016/j.brainres.2011.09.029. Epub 2011 Sep 22. Brain Res. 2011. PMID: 21985959
-
Normalization of NF-κB activity in dorsal root ganglia neurons cultured from diabetic rats reverses neuropathy-linked markers of cellular pathology.Exp Neurol. 2013 Mar;241:169-78. doi: 10.1016/j.expneurol.2012.11.009. Epub 2012 Nov 15. Exp Neurol. 2013. PMID: 23159890
Cited by
-
Neutralization of schwann cell-secreted VEGF is protective to in vitro and in vivo experimental diabetic neuropathy.PLoS One. 2014 Sep 30;9(9):e108403. doi: 10.1371/journal.pone.0108403. eCollection 2014. PLoS One. 2014. PMID: 25268360 Free PMC article.
-
VEGF-B promotes recovery of corneal innervations and trophic functions in diabetic mice.Sci Rep. 2017 Jan 16;7:40582. doi: 10.1038/srep40582. Sci Rep. 2017. PMID: 28091556 Free PMC article.
-
Cisplatin induced sensory neuropathy is prevented by vascular endothelial growth factor-A.Am J Transl Res. 2015 Jun 15;7(6):1032-44. eCollection 2015. Am J Transl Res. 2015. PMID: 26279748 Free PMC article.
-
Animal models as tools to investigate antidiabetic and anti-inflammatory plants.Evid Based Complement Alternat Med. 2012;2012:142087. doi: 10.1155/2012/142087. Epub 2012 Jul 29. Evid Based Complement Alternat Med. 2012. PMID: 22899950 Free PMC article.
-
VEGF-A promotes both pro-angiogenic and neurotrophic capacities for nerve recovery after compressive neuropathy in rats.Mol Neurobiol. 2015 Feb;51(1):240-51. doi: 10.1007/s12035-014-8754-1. Epub 2014 May 28. Mol Neurobiol. 2015. PMID: 24865514
References
-
- Ferrara N, Gerber HP, LeCouter J: The biology of VEGF and its receptors. Nat Med 2003;9:669–676 - PubMed
-
- Sondell M, Kanje M: Postnatal expression of VEGF and its receptor flk-1 in peripheral ganglia. NeuroReport 2001;12:105–108 - PubMed
-
- Sondell M, Lundborg G, Kanje M: Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res 1998;795:44–54 - PubMed
-
- Sondell M, Lundborg G, Kanje M: Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res 1999;846:219–228 - PubMed

