Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation
- PMID: 19934009
- PMCID: PMC2809968
- DOI: 10.2337/db09-0421
Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation
Abstract
Objective: The response of ventromedial hypothalamic (VMH) glucose-inhibited neurons to decreased glucose is impaired under conditions where the counterregulatory response (CRR) to hypoglycemia is impaired (e.g., recurrent hypoglycemia). This suggests a role for glucose-inhibited neurons in the CRR. We recently showed that decreased glucose increases nitric oxide (NO) production in cultured VMH glucose-inhibited neurons. These in vitro data led us to hypothesize that NO release from VMH glucose-inhibited neurons is critical for the CRR.
Research design and methods: The CRR was evaluated in rats and mice in response to acute insulin-induced hypoglycemia and hypoglycemic clamps after modulation of brain NO signaling. The glucose sensitivity of ventromedial nucleus glucose-inhibited neurons was also assessed.
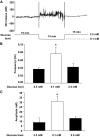
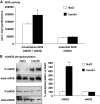
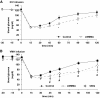
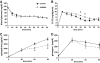
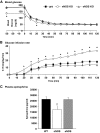
Results: Hypoglycemia increased hypothalamic constitutive NO synthase (NOS) activity and neuronal NOS (nNOS) but not endothelial NOS (eNOS) phosphorylation in rats. Intracerebroventricular and VMH injection of the nonselective NOS inhibitor N(G)-monomethyl-l-arginine (l-NMMA) slowed the recovery to euglycemia after hypoglycemia. VMH l-NMMA injection also increased the glucose infusion rate (GIR) and decreased epinephrine secretion during hyperinsulinemic/hypoglycemic clamp in rats. The GIR required to maintain the hypoglycemic plateau was higher in nNOS knockout than wild-type or eNOS knockout mice. Finally, VMH glucose-inhibited neurons were virtually absent in nNOS knockout mice.
Conclusions: We conclude that VMH NO production is necessary for glucose sensing in glucose-inhibited neurons and full generation of the CRR to hypoglycemia. These data suggest that potentiating NO signaling may improve the defective CRR resulting from recurrent hypoglycemia in patients using intensive insulin therapy.
Figures

References
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986 - PubMed
-
- Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS: Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987;316:1376–1383 - PubMed
-
- Cryer PE: Glucose counterregulation in man. Diabetes 1981;30:261–264 - PubMed
-
- Routh VH, Song Z, Liu X: The role of glucosensing neurons in the detection of hypoglycemia. Diabetes Technol Ther 2004;6:413–421 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

