N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons
- PMID: 19934013
- PMCID: PMC2781051
- DOI: 10.1242/dev.034355
N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons
Abstract
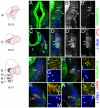
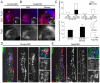
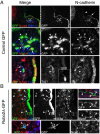
Vertebrate cranial sensory ganglia have a dual origin from the neural crest and ectodermal placodes. In the largest of these, the trigeminal ganglion, Slit1-Robo2 signaling is essential for proper ganglion assembly. Here, we demonstrate a crucial role for the cell adhesion molecule N-cadherin and its interaction with Slit1-Robo2 during gangliogenesis in vivo. A common feature of chick trigeminal and epibranchial ganglia is the expression of N-cadherin and Robo2 on placodal neurons and Slit1 on neural crest cells. Interestingly, N-cadherin localizes to intercellular adherens junctions between placodal neurons during ganglion assembly. Depletion of N-cadherin causes loss of proper ganglion coalescence, similar to that observed after loss of Robo2, suggesting that the two pathways might intersect. Consistent with this possibility, blocking or augmenting Slit-Robo signaling modulates N-cadherin protein expression on the placodal cell surface concomitant with alteration in placodal adhesion. Lack of an apparent change in total N-cadherin mRNA or protein levels suggests post-translational regulation. Co-expression of N-cadherin with dominant-negative Robo abrogates the Robo2 loss-of-function phenotype of dispersed ganglia, whereas loss of N-cadherin reverses the aberrant aggregation induced by increased Slit-Robo expression. Our study suggests a novel mechanism whereby N-cadherin acts in concert with Slit-Robo signaling in mediating the placodal cell adhesion required for proper gangliogenesis.
Figures
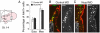

Similar articles
-
Robo2-Slit1 dependent cell-cell interactions mediate assembly of the trigeminal ganglion.Nat Neurosci. 2008 Mar;11(3):269-76. doi: 10.1038/nn2051. Epub 2008 Feb 17. Nat Neurosci. 2008. PMID: 18278043
-
N-cadherin facilitates trigeminal sensory neuron outgrowth and target tissue innervation.Development. 2025 May 1;152(9):dev204369. doi: 10.1242/dev.204369. Epub 2025 May 1. Development. 2025. PMID: 40260574 Free PMC article.
-
Cadherin-7 mediates proper neural crest cell-placodal neuron interactions during trigeminal ganglion assembly.Genesis. 2019 Jan;57(1):e23264. doi: 10.1002/dvg.23264. Epub 2018 Dec 24. Genesis. 2019. PMID: 30461190 Free PMC article.
-
Molecular and tissue interactions governing induction of cranial ectodermal placodes.Dev Biol. 2009 Aug 15;332(2):189-95. doi: 10.1016/j.ydbio.2009.05.572. Epub 2009 Jun 2. Dev Biol. 2009. PMID: 19500565 Free PMC article. Review.
-
Cadherin dynamics during neural crest cell ontogeny.Prog Mol Biol Transl Sci. 2013;116:291-315. doi: 10.1016/B978-0-12-394311-8.00013-3. Prog Mol Biol Transl Sci. 2013. PMID: 23481200 Review.
Cited by
-
Transgenic animal models of congenital diaphragmatic hernia: a comprehensive overview of candidate genes and signaling pathways.Pediatr Surg Int. 2020 Sep;36(9):991-997. doi: 10.1007/s00383-020-04705-0. Epub 2020 Jun 26. Pediatr Surg Int. 2020. PMID: 32591848 Free PMC article. Review.
-
Diversity in the molecular and cellular strategies of epithelium-to-mesenchyme transitions: Insights from the neural crest.Cell Adh Migr. 2010 Jul-Sep;4(3):458-82. doi: 10.4161/cam.4.3.12501. Epub 2010 Jul 27. Cell Adh Migr. 2010. PMID: 20559020 Free PMC article.
-
An effective assay for high cellular resolution time-lapse imaging of sensory placode formation and morphogenesis.BMC Neurosci. 2011 May 9;12:37. doi: 10.1186/1471-2202-12-37. BMC Neurosci. 2011. PMID: 21554727 Free PMC article.
-
Neural crest and placode interaction during the development of the cranial sensory system.Dev Biol. 2014 May 1;389(1):28-38. doi: 10.1016/j.ydbio.2014.01.021. Epub 2014 Jan 31. Dev Biol. 2014. PMID: 24491819 Free PMC article. Review.
-
Slits affect the timely migration of neural crest cells via Robo receptor.Dev Dyn. 2012 Aug;241(8):1274-88. doi: 10.1002/dvdy.23817. Epub 2012 Jun 23. Dev Dyn. 2012. PMID: 22689303 Free PMC article.
References
-
- Akitaya T., Bronner-Fraser M. (1992). Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev. Dyn. 194, 12-20 - PubMed
-
- Baker C. V. (2005). Neural crest and cranial ectodermal placodes. In Developmental Neurobiology (ed. Jacobson M. S. R. a. M.), pp. 67-127 New York: Kluwer Academic/Plenum Publishers;
-
- Begbie J., Graham A. (2001). Integration between the epibranchial placodes and the hindbrain. Science 294, 595-598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

