Germinal center reutilization by newly activated B cells
- PMID: 19934021
- PMCID: PMC2806468
- DOI: 10.1084/jem.20091225
Germinal center reutilization by newly activated B cells
Abstract
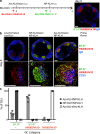
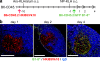
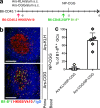
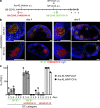
Germinal centers (GCs) are specialized structures in which B lymphocytes undergo clonal expansion, class switch recombination, somatic hypermutation, and affinity maturation. Although these structures were previously thought to contain a limited number of isolated B cell clones, recent in vivo imaging studies revealed that they are in fact dynamic and appear to be open to their environment. We demonstrate that B cells can colonize heterologous GCs. Invasion of primary GCs after subsequent immunization is most efficient when T cell help is shared by the two immune responses; however, it also occurs when the immune responses are entirely unrelated. We conclude that GCs are dynamic anatomical structures that can be reutilized by newly activated B cells during immune responses.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

