GATA-3 is required for early T lineage progenitor development
- PMID: 19934022
- PMCID: PMC2806453
- DOI: 10.1084/jem.20090934
GATA-3 is required for early T lineage progenitor development
Abstract
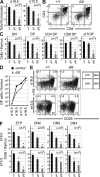
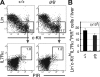
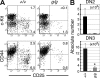
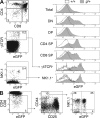
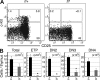
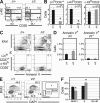
Most T lymphocytes appear to arise from very rare early T lineage progenitors (ETPs) in the thymus, but the transcriptional programs that specify ETP generation are not completely known. The transcription factor GATA-3 is required for the development of T lymphocytes at multiple late differentiation steps as well as for the development of thymic natural killer cells. However, a role for GATA-3 before the double-negative (DN) 3 stage of T cell development has to date been obscured both by the developmental heterogeneity of DN1 thymocytes and the paucity of ETPs. We provide multiple lines of in vivo evidence through the analysis of T cell development in Gata3 hypomorphic mutant embryos, in irradiated mice reconstituted with Gata3 mutant hematopoietic cells, and in mice conditionally ablated for the Gata3 gene to show that GATA-3 is required for ETP generation. We further show that Gata3 loss does not affect hematopoietic stem cells or multipotent hematopoietic progenitors. Finally, we demonstrate that Gata3 mutant lymphoid progenitors exhibit neither increased apoptosis nor diminished cell-cycle progression. Thus, GATA-3 is required for the cell-autonomous development of the earliest characterized thymic T cell progenitors.
Figures


Similar articles
-
From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation.Immunol Rev. 2010 Nov;238(1):110-25. doi: 10.1111/j.1600-065X.2010.00954.x. Immunol Rev. 2010. PMID: 20969588 Free PMC article. Review.
-
Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus.Eur J Immunol. 1999 Jun;29(6):1912-8. doi: 10.1002/(SICI)1521-4141(199906)29:06<1912::AID-IMMU1912>3.0.CO;2-D. Eur J Immunol. 1999. PMID: 10382753
-
Involvement of CCR9 at multiple stages of adult T lymphopoiesis.J Leukoc Biol. 2008 Jan;83(1):156-64. doi: 10.1189/jlb.0607423. Epub 2007 Oct 2. J Leukoc Biol. 2008. PMID: 17911179
-
The transcription factor Lyl-1 regulates lymphoid specification and the maintenance of early T lineage progenitors.Nat Immunol. 2012 Jul 8;13(8):761-9. doi: 10.1038/ni.2365. Nat Immunol. 2012. PMID: 22772404 Free PMC article.
-
GATA-3 - not just for Th2 cells anymore.Cell Mol Immunol. 2007 Feb;4(1):15-29. Cell Mol Immunol. 2007. PMID: 17349208 Review.
Cited by
-
GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle entry.Blood. 2012 Mar 8;119(10):2242-51. doi: 10.1182/blood-2011-07-366070. Epub 2012 Jan 20. Blood. 2012. PMID: 22267605 Free PMC article.
-
FLT3 mutations in early T-cell precursor ALL characterize a stem cell like leukemia and imply the clinical use of tyrosine kinase inhibitors.PLoS One. 2013;8(1):e53190. doi: 10.1371/journal.pone.0053190. Epub 2013 Jan 24. PLoS One. 2013. PMID: 23359050 Free PMC article.
-
Multilayered specification of the T-cell lineage fate.Immunol Rev. 2010 Nov;238(1):150-68. doi: 10.1111/j.1600-065X.2010.00964.x. Immunol Rev. 2010. PMID: 20969591 Free PMC article. Review.
-
An NK and T cell enhancer lies 280 kilobase pairs 3' to the gata3 structural gene.Mol Cell Biol. 2011 May;31(9):1894-904. doi: 10.1128/MCB.05065-11. Epub 2011 Mar 7. Mol Cell Biol. 2011. PMID: 21383068 Free PMC article.
-
Developmental gene networks: a triathlon on the course to T cell identity.Nat Rev Immunol. 2014 Aug;14(8):529-45. doi: 10.1038/nri3702. Nat Rev Immunol. 2014. PMID: 25060579 Free PMC article. Review.
References
-
- Adolfsson J., Månsson R., Buza-Vidas N., Hultquist A., Liuba K., Jensen C.T., Bryder D., Yang L., Borge O.J., Thoren L.A., et al. 2005. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential: a revised road map for adult blood lineage commitment. Cell. 121:295–306 10.1016/j.cell.2005.02.013 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

