The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish
- PMID: 19934034
- PMCID: PMC2781060
- DOI: 10.1073/pnas.0911632106
The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish
Abstract
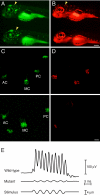
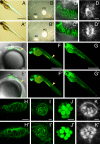
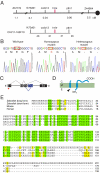
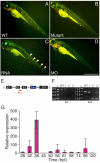
Little is known about the proteins that mediate mechanoelectrical transduction, the process by which acoustic and accelerational stimuli are transformed by hair cells of the inner ear into electrical signals. In our search for molecules involved in mechanotransduction, we discovered a line of deaf and uncoordinated zebrafish with defective hair-cell function. The hair cells of mutant larvae fail to incorporate fluorophores that normally traverse the transduction channels and their ears lack microphonic potentials in response to vibratory stimuli. Hair cells in the posterior lateral lines of mutants contain numerous lysosomes and have short, disordered hair bundles. Their stereocilia lack two components of the transduction apparatus, tip links and insertional plaques. Positional cloning revealed an early frameshift mutation in tmie, the zebrafish ortholog of the mammalian gene transmembrane inner ear. The mutant line therefore affords us an opportunity to investigate the role of the corresponding protein in mechanoelectrical transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Disruption of tmc1/2a/2b Genes in Zebrafish Reveals Subunit Requirements in Subtypes of Inner Ear Hair Cells.J Neurosci. 2020 Jun 3;40(23):4457-4468. doi: 10.1523/JNEUROSCI.0163-20.2020. Epub 2020 May 5. J Neurosci. 2020. PMID: 32371604 Free PMC article.
-
NompC TRP channel required for vertebrate sensory hair cell mechanotransduction.Science. 2003 Jul 4;301(5629):96-9. doi: 10.1126/science.1084370. Epub 2003 Jun 12. Science. 2003. PMID: 12805553
-
Subunits of the mechano-electrical transduction channel, Tmc1/2b, require Tmie to localize in zebrafish sensory hair cells.PLoS Genet. 2019 Feb 6;15(2):e1007635. doi: 10.1371/journal.pgen.1007635. eCollection 2019 Feb. PLoS Genet. 2019. PMID: 30726219 Free PMC article.
-
The genetics of hair-cell function in zebrafish.J Neurogenet. 2017 Sep;31(3):102-112. doi: 10.1080/01677063.2017.1342246. Epub 2017 Jul 13. J Neurogenet. 2017. PMID: 28705044 Free PMC article. Review.
-
Hair cells, plasma membrane Ca²⁺ ATPase and deafness.Int J Biochem Cell Biol. 2012 May;44(5):679-83. doi: 10.1016/j.biocel.2012.02.006. Epub 2012 Feb 13. Int J Biochem Cell Biol. 2012. PMID: 22349217 Review.
Cited by
-
Transcriptomic Analyses of Inner Ear Sensory Epithelia in Zebrafish.Anat Rec (Hoboken). 2020 Mar;303(3):527-543. doi: 10.1002/ar.24331. Epub 2019 Dec 28. Anat Rec (Hoboken). 2020. PMID: 31883312 Free PMC article.
-
Distinct functions of TMC channels: a comparative overview.Cell Mol Life Sci. 2019 Nov;76(21):4221-4232. doi: 10.1007/s00018-019-03214-1. Epub 2019 Oct 4. Cell Mol Life Sci. 2019. PMID: 31584127 Free PMC article. Review.
-
The how and why of identifying the hair cell mechano-electrical transduction channel.Pflugers Arch. 2015 Jan;467(1):73-84. doi: 10.1007/s00424-014-1606-z. Epub 2014 Sep 23. Pflugers Arch. 2015. PMID: 25241775 Free PMC article. Review.
-
Complexes of vertebrate TMC1/2 and CIB2/3 proteins form hair-cell mechanotransduction cation channels.bioRxiv [Preprint]. 2024 Jul 5:2023.05.26.542533. doi: 10.1101/2023.05.26.542533. bioRxiv. 2024. Update in: Elife. 2025 Jan 08;12:RP89719. doi: 10.7554/eLife.89719. PMID: 37398045 Free PMC article. Updated. Preprint.
-
Gene miles-apart is required for formation of otic vesicle and hair cells in zebrafish.Cell Death Dis. 2013 Oct 31;4(10):e900. doi: 10.1038/cddis.2013.432. Cell Death Dis. 2013. PMID: 24176858 Free PMC article.
References
-
- Hudspeth AJ. How the ear's works work. Nature. 1989;341:397–404. - PubMed
-
- Petit C, Levilliers J, Hardelin JP. Molecular genetics of hearing loss. Annu Rev Genet. 2001;35:589–646. - PubMed
-
- Dooley K, Zon LI. Zebrafish: A model system for the study of human disease. Curr Opin Genet Dev. 2000;10:252–256. - PubMed
-
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, danio rerio. J Comp Neurol. 1996;365:113–128. - PubMed
-
- Blaxter JHS, Fuiman LA. In: The Mechanosensory Lateral Line: Neurobiology and Evolution. Coombs S, Görner P, Münz H, editors. New York: Springer; 1989. pp. 481–499.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

