A nonprotein thermal hysteresis-producing xylomannan antifreeze in the freeze-tolerant Alaskan beetle Upis ceramboides
- PMID: 19934038
- PMCID: PMC2787118
- DOI: 10.1073/pnas.0909872106
A nonprotein thermal hysteresis-producing xylomannan antifreeze in the freeze-tolerant Alaskan beetle Upis ceramboides
Abstract
Thermal hysteresis (TH), a difference between the melting and freezing points of a solution that is indicative of the presence of large-molecular-mass antifreezes (e.g., antifreeze proteins), has been described in animals, plants, bacteria, and fungi. Although all previously described TH-producing biomolecules are proteins, most thermal hysteresis factors (THFs) have not yet been structurally characterized, and none have been characterized from a freeze-tolerant animal. We isolated a highly active THF from the freeze-tolerant beetle, Upis ceramboides, by means of ice affinity. Amino acid chromatographic analysis, polyacrylamide gel electrophoresis, UV-Vis spectrophotometry, and NMR spectroscopy indicated that the THF contained little or no protein, yet it produced 3.7 +/- 0.3 degrees C of TH at 5 mg/ml, comparable to that of the most active insect antifreeze proteins. Compositional and structural analyses indicated that this antifreeze contains a beta-mannopyranosyl-(1-->4) beta-xylopyranose backbone and a fatty acid component, although the lipid may not be covalently linked to the saccharide. Consistent with the proposed structure, treatment with endo-beta-(1-->4)xylanase ablated TH activity. This xylomannan is the first TH-producing antifreeze isolated from a freeze-tolerant animal and the first in a new class of highly active THFs that contain little or no protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
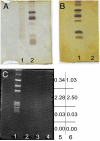



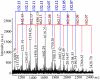
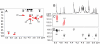

References
-
- DeVries AL, Komatsu SK, Feeney RE. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J Biol Chem. 1970;245:2901–2908. - PubMed
-
- DeVries AL. Antifreeze glycopeptides and peptides: Interactions with ice and water. Methods Enzymol. 1986;127:293–303. - PubMed
-
- DeVries AL. In: Barnes B, Carey H, editors. Life in the Cold: Evolution, Mechanisms, Adaptation and Application; Twelfth International Hibernation Symposium; Fairbanks: Institute of Arctic Biology; 2004. pp. 307–315.
-
- Venketesh S, Dayananda C. Properties, potentials, and prospects of antifreeze proteins. Crit Rev Biotechnol. 2008;28:57–82. - PubMed
-
- Davies PL, Sykes BD. Antifreeze proteins. Curr Opin Struct Biol. 1997;7:828–834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

