Synergy between bacterial infection and genetic predisposition in intestinal dysplasia
- PMID: 19934041
- PMCID: PMC2791635
- DOI: 10.1073/pnas.0911797106
Synergy between bacterial infection and genetic predisposition in intestinal dysplasia
Abstract
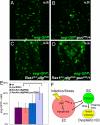
Accumulating evidence suggests that hyperproliferating intestinal stem cells (SCs) and progenitors drive cancer initiation, maintenance, and metastasis. In addition, chronic inflammation and infection have been increasingly recognized for their roles in cancer. Nevertheless, the mechanisms by which bacterial infections can initiate SC-mediated tumorigenesis remain elusive. Using a Drosophila model of gut pathogenesis, we show that intestinal infection with Pseudomonas aeruginosa, a human opportunistic bacterial pathogen, activates the c-Jun N-terminal kinase (JNK) pathway, a hallmark of the host stress response. This, in turn, causes apoptosis of enterocytes, the largest class of differentiated intestinal cells, and promotes a dramatic proliferation of SCs and progenitors that serves as a homeostatic compensatory mechanism to replenish the apoptotic enterocytes. However, we find that this homeostatic mechanism can lead to massive over-proliferation of intestinal cells when infection occurs in animals with a latent oncogenic form of the Ras1 oncogene. The affected intestines develop excess layers of cells with altered apicobasal polarity reminiscent of dysplasia, suggesting that infection can directly synergize with the genetic background in predisposed individuals to initiate SC-mediated tumorigenesis. Our results provide a framework for the study of intestinal bacterial infections and their effects on undifferentiated and mature enteric epithelial cells in the initial stages of intestinal cancer. Assessment of progenitor cell responses to pathogenic intestinal bacteria could provide a measure of predisposition for apoptotic enterocyte-assisted intestinal dysplasias in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
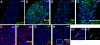
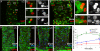
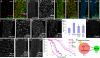
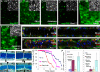
References
-
- Loktionov A. Cell exfoliation in the human colon: Myth, reality, and implications for colorectal cancer screening. Int J Cancer. 2007;120:2281–2289. - PubMed
-
- Lakatos PL, Fischer S, Lakatos L, Gal I, Papp J. Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: Pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take “toll”? World J Gastroenterol. 2006;12:1829–1841. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

