Using marginal structural measurement-error models to estimate the long-term effect of antiretroviral therapy on incident AIDS or death
- PMID: 19934191
- PMCID: PMC2800300
- DOI: 10.1093/aje/kwp329
Using marginal structural measurement-error models to estimate the long-term effect of antiretroviral therapy on incident AIDS or death
Abstract
To estimate the net effect of imperfectly measured highly active antiretroviral therapy on incident acquired immunodeficiency syndrome or death, the authors combined inverse probability-of-treatment-and-censoring weighted estimation of a marginal structural Cox model with regression-calibration methods. Between 1995 and 2007, 950 human immunodeficiency virus-positive men and women were followed in 2 US cohort studies. During 4,054 person-years, 374 initiated highly active antiretroviral therapy, 211 developed acquired immunodeficiency syndrome or died, and 173 dropped out. Accounting for measured confounders and determinants of dropout, the weighted hazard ratio for acquired immunodeficiency syndrome or death comparing use of highly active antiretroviral therapy in the prior 2 years with no therapy was 0.36 (95% confidence limits: 0.21, 0.61). This association was relatively constant over follow-up (P = 0.19) and stronger than crude or adjusted hazard ratios of 0.75 and 0.95, respectively. Accounting for measurement error in reported exposure using external validation data on 331 men and women provided a hazard ratio of 0.17, with bias shifted from the hazard ratio to the estimate of precision as seen by the 2.5-fold wider confidence limits (95% confidence limits: 0.06, 0.43). Marginal structural measurement-error models can simultaneously account for 3 major sources of bias in epidemiologic research: validated exposure measurement error, measured selection bias, and measured time-fixed and time-varying confounding.
Figures
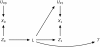
References
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–733. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy [see comments] N Engl J Med. 1997;337(11):734–739. - PubMed
-
- Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet. 1998;351(9102):543–549. - PubMed
-
- Miettinen OS. The need for randomization in the study of intended effects. Stat Med. 1983;2(2):267–271. - PubMed
-
- Greenland S, Robins JM. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol. 1986;15(3):413–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI037984/AI/NIAID NIH HHS/United States
- UO1-AI-34994/AI/NIAID NIH HHS/United States
- UO1-AI-34989/AI/NIAID NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- UO1-AI-35042/AI/NIAID NIH HHS/United States
- UO1-AI-35040/AI/NIAID NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- R01-AA-017594/AA/NIAAA NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- UO1-HD-32632/HD/NICHD NIH HHS/United States
- UO1-AI-37984/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- RR024131/RR/NCRR NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 AI037613/AI/NIAID NIH HHS/United States
- UO1-AI-35041/AI/NIAID NIH HHS/United States
- R03 AI071763/AI/NIAID NIH HHS/United States
- M01 RR000722/RR/NCRR NIH HHS/United States
- P30-AI-50410/AI/NIAID NIH HHS/United States
- UO1-AI-35004/AI/NIAID NIH HHS/United States
- R01 AA017594/AA/NIAAA NIH HHS/United States
- UO1-AI-34993/AI/NIAID NIH HHS/United States
- R03-AI-071763/AI/NIAID NIH HHS/United States
- UO1-AI-35039/AI/NIAID NIH HHS/United States
- UO1-AI-42590/AI/NIAID NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- 5-MO1-RR-00722/RR/NCRR NIH HHS/United States
- UO1-AI-31834/AI/NIAID NIH HHS/United States
- UO1-AI-35043/AI/NIAID NIH HHS/United States
- UO1-AI-37613/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

