Toll-dependent antimicrobial responses in Drosophila larval fat body require Spätzle secreted by haemocytes
- PMID: 19934223
- PMCID: PMC2787462
- DOI: 10.1242/jcs.049155
Toll-dependent antimicrobial responses in Drosophila larval fat body require Spätzle secreted by haemocytes
Abstract
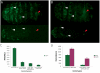
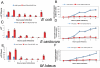
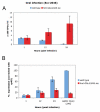
In Drosophila, the humoral response characterised by the synthesis of antimicrobial peptides (AMPs) in the fat body (the equivalent of the mammalian liver) and the cellular response mediated by haemocytes (blood cells) engaged in phagocytosis represent two major reactions that counter pathogens. Although considerable analysis has permitted the elucidation of mechanisms pertaining to the two responses individually, the mechanism of their coordination has been unclear. To characterise the signals with which infection might be communicated between blood cells and fat body, we ablated circulating haemocytes and defined the parameters of AMP gene activation in larvae. We found that targeted ablation of blood cells influenced the levels of AMP gene expression in the fat body following both septic injury and oral infection. Expression of the AMP gene drosomycin (a Toll target) was blocked when expression of the Toll ligand Spätzle was knocked down in haemocytes. These results show that in larvae, integration of the two responses in a systemic reaction depend on the production of a cytokine (spz), a process that strongly parallels the mammalian immune response.
Figures
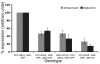
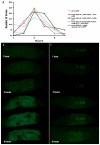
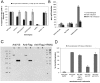
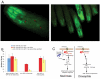
Similar articles
-
New insights into Drosophila larval haemocyte functions through genome-wide analysis.Cell Microbiol. 2005 Mar;7(3):335-50. doi: 10.1111/j.1462-5822.2004.00462.x. Cell Microbiol. 2005. PMID: 15679837
-
TmDorX2 positively regulates antimicrobial peptides in Tenebrio molitor gut, fat body, and hemocytes in response to bacterial and fungal infection.Sci Rep. 2019 Nov 14;9(1):16878. doi: 10.1038/s41598-019-53497-4. Sci Rep. 2019. PMID: 31728023 Free PMC article.
-
A Toll-Spätzle pathway in the tobacco hornworm, Manduca sexta.Insect Biochem Mol Biol. 2012 Jul;42(7):514-24. doi: 10.1016/j.ibmb.2012.03.009. Epub 2012 Apr 6. Insect Biochem Mol Biol. 2012. PMID: 22516181 Free PMC article.
-
The host defense of Drosophila melanogaster.Annu Rev Immunol. 2007;25:697-743. doi: 10.1146/annurev.immunol.25.022106.141615. Annu Rev Immunol. 2007. PMID: 17201680 Review.
-
How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions.Curr Opin Microbiol. 2002 Feb;5(1):102-10. doi: 10.1016/s1369-5274(02)00294-1. Curr Opin Microbiol. 2002. PMID: 11834378 Review.
Cited by
-
Ehrlichia chaffeensis replication sites in adult Drosophila melanogaster.Int J Med Microbiol. 2013 Jan;303(1):40-9. doi: 10.1016/j.ijmm.2012.12.002. Epub 2013 Jan 8. Int J Med Microbiol. 2013. PMID: 23306065 Free PMC article.
-
Spatially Restricted Regulation of Spätzle/Toll Signaling during Cell Competition.Dev Cell. 2018 Sep 24;46(6):706-719.e5. doi: 10.1016/j.devcel.2018.08.001. Epub 2018 Aug 23. Dev Cell. 2018. PMID: 30146479 Free PMC article.
-
Regulating metabolism to shape immune function: Lessons from Drosophila.Semin Cell Dev Biol. 2023 Mar 30;138:128-141. doi: 10.1016/j.semcdb.2022.04.002. Epub 2022 Apr 16. Semin Cell Dev Biol. 2023. PMID: 35440411 Free PMC article. Review.
-
Immunometabolic regulation during the presence of microorganisms and parasitoids in insects.Front Immunol. 2023 Sep 25;14:905467. doi: 10.3389/fimmu.2023.905467. eCollection 2023. Front Immunol. 2023. PMID: 37818375 Free PMC article. Review.
-
Drosophila as a Genetic Model for Hematopoiesis.Genetics. 2019 Feb;211(2):367-417. doi: 10.1534/genetics.118.300223. Genetics. 2019. PMID: 30733377 Free PMC article. Review.
References
-
- Agaisse, H., Petersen, U. M., Boutros, M., Mathey-Prevot, B. and Perrimon, N. (2003). Signalling role of haemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 5, 441-450. - PubMed
-
- Akira, S., Uematsu, S. and Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell 124, 783-801. - PubMed
-
- Anderson, K. V. and Nüsslein-Volhard, C. (1984). Genetic analysis of dorsal-ventral embryonic pattern in Drosophila. In Pattern Formation: A Primer in Developmental Biology (ed. G. Malacinski and S. V. Bryant), pp. 269-289. New York: Macmillan.
-
- Anderson, K. V., Jürgens, G., Nüsslein-Volhard, C. (1985) Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42, 779-789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

