Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer
- PMID: 19934294
- PMCID: PMC2898570
- DOI: 10.1158/1078-0432.CCR-09-1249
Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer
Abstract
Purpose: The mammalian target of rapamycin (mTOR) kinase acts downstream of phosphoinositide 3-kinase/Akt to regulate cellular growth, metabolism, and cytoskeleton. Because approximately 60% of sporadic colorectal cancers (CRC) exhibit high levels of activated Akt, we determined whether downstream mTOR signaling pathway components are overexpressed and activated in CRCs.
Experimental design: HCT116, KM20, Caco-2, and SW480 human CRC cells were used to determine the effects of pharmacologic (using rapamycin) or genetic (using RNAi) blockade of mTOR signaling on cell proliferation, apoptosis, cell cycle progression, and subcutaneous growth in vivo.
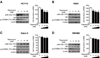
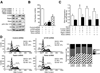
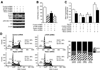
Results: We show that the mTOR complex proteins mTOR, Raptor, and Rictor are overexpressed in CRC. Treatment with rapamycin significantly decreased proliferation of certain CRC cell lines (rapamycin sensitive), whereas other cell lines were resistant to its effects (rapamycin resistant). Transient siRNA-mediated knockdown of the mTORC2 protein, Rictor, significantly decreased proliferation of both rapamycin-sensitive and rapamycin-resistant CRC cells. Stable shRNA-mediated knockdown of both mTORC1 and mTORC2 decreased proliferation, increased apoptosis, and attenuated cell cycle progression in rapamycin-sensitive CRCs. Moreover, stable knockdown of both mTORC1 and mTORC2 decreased proliferation and attenuated cell cycle progression, whereas only mTORC2 knockdown increased apoptosis in rapamycin-resistant CRCs. Finally, knockdown of both mTORC1 and mTORC2 inhibited growth of rapamycin-sensitive and rapamycin-resistant CRCs in vivo when implanted as tumor xenografts.
Conclusions: Targeted inhibition of the mTORC2 protein, Rictor, leads to growth inhibition and induces apoptosis in both rapamycin-sensitive and rapamycin-resistant CRCs, suggesting that selective targeting of mTORC2 may represent a novel therapeutic strategy for treatment of CRC.
Figures



References
-
- Philp AJ, Campbell IG, Leet C, et al. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. - PubMed
-
- Roy HK, Olusola BF, Clemens DL, et al. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. - PubMed
-
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. - PubMed
-
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. - PubMed
-
- Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

