Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model
- PMID: 19934299
- PMCID: PMC2787715
- DOI: 10.1158/1078-0432.CCR-09-1292
Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model
Abstract
Purpose: Preexisting antiviral antibodies in cancer patients can quickly neutralize oncolytic measles virus (MV) and decrease its antitumor potency. In contrast to "naked" viruses, cell-associated viruses are protected from antibody neutralization. Hence, we hypothesized that measles virotherapy of ovarian cancer in measles-immune mice might be superior if MV-infected mesenchymal stem cell (MSC) carriers are used.
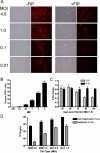
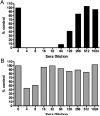
Experimental design: Antimeasles antibodies titers in ovarian cancer patients were determined. The protection of MV by MSC from antimeasles antibodies, the in vivo biodistribution profiles, and tumor infiltration capability of MSC were determined. Measles-naïve or immune tumor-bearing mice were treated with naked virus or MSC-associated virus and mice survivals were compared.
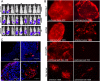
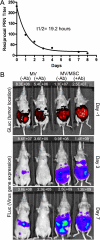
Results: MSC transferred MV infection to target cells via cell-to-cell heterofusion and induced syncytia formation in the presence of high titers of antimeasles antibody, at levels that completely inactivated naked virus. Athymic mice bearing i.p. human SKOV3ip.1 ovarian tumor xenografts passively immunized with measles-immune human serum were treated with saline, naked MV, or MV-infected MSC. Bioluminescent and fluorescent imaging data indicated that i.p. administered MSC localized to peritoneal tumors, infiltrated into the tumor parenchyma, and transferred virus infection to tumors in measles naïve and passively immunized mice. Survival of the measles-immune mice was significantly enhanced by treatment with MV-infected MSC. In contrast, survivals of passively immunized mice were not prolonged by treatment with naked virus or uninfected MSC.
Conclusions: MSC should be used as carriers of MV for intraperitoneal virotherapy in measles-immune ovarian cancer patients.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Rocconi RP, Numnum TM, Stoff-Khalili M, et al. Targeted gene therapy for ovarian cancer. Curr Gene Ther. 2005;5:643–653. - PubMed
-
- Kimball KJ, Numnum TM, Rocconi RP, Alvarez RD. Gene therapy for ovarian cancer. Curr Oncol Rep. 2006;8:441–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

