Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity
- PMID: 19934310
- PMCID: PMC2828615
- DOI: 10.1158/0008-5472.CAN-09-1605
Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity
Abstract
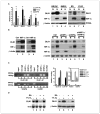
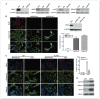
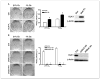
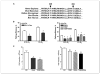
Reduced oxygenation, or hypoxia, inhibits differentiation and facilitates stem cell maintenance. Hypoxia commonly occurs in solid tumors and promotes malignant progression. Hypoxic tumors are aggressive and exhibit stem cell-like characteristics. It remains unclear, however, whether and how hypoxia regulates cancer cell differentiation and maintains cancer cell stemness. Here, we show that hypoxia increases the expression of the stem cell gene DLK1, or delta-like 1 homologue (Drosophila), in neuronal tumor cells. Inhibition of DLK1 enhances spontaneous differentiation, decreases clonogenicity, and reduces in vivo tumor growth. Overexpression of DLK1 inhibits differentiation and enhances tumorigenic potentials. We further show that the DLK1 cytoplasmic domain, especially Tyrosine339 and Serine355, is required for maintaining both clonogenicity and tumorigenicity. Because elevated DLK1 expression is found in many tumor types, our observations suggest that hypoxia and DLK1 may constitute an important stem cell pathway for the regulation of cancer stem cell-like functionality and tumorigenicity.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Interaction of delta-like 1 homolog (Drosophila) with prohibitins and its impact on tumor cell clonogenicity.Mol Cancer Res. 2014 Jan;12(1):155-64. doi: 10.1158/1541-7786.MCR-13-0360. Epub 2013 Nov 18. Mol Cancer Res. 2014. PMID: 24249679 Free PMC article.
-
DLK1, delta-like 1 homolog (Drosophila), regulates tumor cell differentiation in vivo.Cancer Lett. 2012 May 1;318(1):26-33. doi: 10.1016/j.canlet.2011.11.032. Epub 2011 Dec 3. Cancer Lett. 2012. PMID: 22142700 Free PMC article.
-
Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells.Oncol Rep. 2013 Oct;30(4):1869-77. doi: 10.3892/or.2013.2643. Epub 2013 Jul 30. Oncol Rep. 2013. PMID: 23900747
-
Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness.J Histochem Cytochem. 2022 Jan;70(1):17-28. doi: 10.1369/00221554211048951. Epub 2021 Oct 4. J Histochem Cytochem. 2022. PMID: 34606325 Free PMC article. Review.
-
Delta-like homologue 1 and its role in the bone marrow niche and hematologic malignancies.Clin Lymphoma Myeloma Leuk. 2014 Dec;14(6):451-5. doi: 10.1016/j.clml.2014.06.019. Epub 2014 Jun 23. Clin Lymphoma Myeloma Leuk. 2014. PMID: 25066037 Review.
Cited by
-
Impact of the hypoxic tumor microenvironment on the regulation of cancer stem cell characteristics.Cancer Biol Ther. 2010 Jun 15;9(12):949-56. doi: 10.4161/cbt.9.12.12347. Epub 2010 Jun 11. Cancer Biol Ther. 2010. PMID: 20581454 Free PMC article. Review.
-
Hypoxic Induction of Vasorin Regulates Notch1 Turnover to Maintain Glioma Stem-like Cells.Cell Stem Cell. 2018 Jan 4;22(1):104-118.e6. doi: 10.1016/j.stem.2017.10.005. Epub 2017 Nov 30. Cell Stem Cell. 2018. PMID: 29198941 Free PMC article.
-
Niche-derived soluble DLK1 promotes glioma growth.Neoplasia. 2020 Dec;22(12):689-701. doi: 10.1016/j.neo.2020.10.005. Epub 2020 Oct 23. Neoplasia. 2020. PMID: 33142235 Free PMC article.
-
Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration.J Biol Chem. 2012 Mar 23;287(13):10355-10367. doi: 10.1074/jbc.M111.312751. Epub 2012 Feb 1. J Biol Chem. 2012. PMID: 22298767 Free PMC article.
-
Compositional analysis of walnut lipid extracts and properties as an anti-cancer stem cell regulator via suppression of the self-renewal capacity.Food Sci Biotechnol. 2016 Apr 30;25(2):623-629. doi: 10.1007/s10068-016-0087-6. eCollection 2016. Food Sci Biotechnol. 2016. PMID: 30263315 Free PMC article.
References
-
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells-perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. - PubMed
-
- Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008;68:4018–21. - PubMed
-
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. - PubMed
-
- Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

