A stretch of 17 amino acids in the prosaposin C terminus is critical for its binding to sortilin and targeting to lysosomes
- PMID: 19934382
- PMCID: PMC2825494
- DOI: 10.1369/jhc.2009.955203
A stretch of 17 amino acids in the prosaposin C terminus is critical for its binding to sortilin and targeting to lysosomes
Abstract
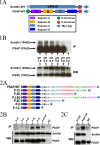
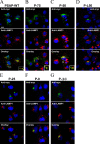
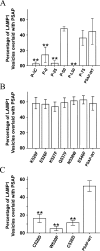
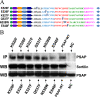
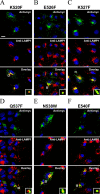
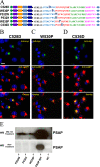
Prosaposin, the precursor of four lysosomal cofactors required for the hydrolysis of sphingolipids, is transported to the lysosomes via the alternative receptor, sortilin. In this study, we identified a specific domain of 17 amino acids within the C terminus of prosaposin involved in binding to this sorting receptor. We generated six prosaposin deletion constructs and examined the effect of truncation by coimmunoprecipitation and confocal microscopy. The experiments revealed that the first half of the prosaposin C terminus (aa 524-540), containing a saposin-like motif, was required and necessary to bind sortilin and to transport it to the lysosomes. Based on this result, we introduced twelve site-directed point mutations within the first half of the C terminus. Although the interaction of prosaposin with sortilin was pH dependent, the mutation of hydrophilic amino acids that usually modulate pH-dependent protein interactions did not affect the binding of prosaposin to sortilin. Conversely, a tryptophan (W530) and two cysteines (C528 and C536) were essential for its interaction with sortilin and for its transport to the lysosomes. In conclusion, our investigation demonstrates that a saposin-like motif within the first half of the prosaposin C terminus contains the sortilin recognition site.
Figures

References
-
- Banares-Hidalgo A, Bolanos-Gutierrez A, Gil F, Cabre EJ, Perez-Gil J, Estrada P (2008) Self-aggregation of a recombinant form of the propeptide NH2-terminal of the precursor of pulmonary surfactant protein SP-B: a conformational study. J Ind Microbiol Biotechnol 35:1367–1376 - PubMed
-
- Betts MJ, Russell RB (2003) Amino acid properties and consequences of substitutions. In Barnes MR, Gray IC, eds. Bioinformatics for Geneticists (hierarchical exotoxicology mini series). Chichester, UK, John Wiley and Sons, 289–316
-
- Canuel M, Bhattacharyya N, Balbis A, Yuan L, Morales CR (2009) Sortilin and prosaposin localize to detergent-resistant membrane microdomains. Exp Cell Res 315:240–247 - PubMed
-
- Canuel M, Lefrancois S, Zeng J, Morales CR (2008) AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem Biophys Res Commun 366:724–730 - PubMed
-
- Coffey JW, De Duve C (1968) Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem 243:3255–3263 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

