Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations
- PMID: 19934397
- PMCID: PMC2835426
- DOI: 10.1124/jpet.109.161927
Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations
Abstract
Milk thistle (Silybum marianum) is a popular herbal product used for hepatoprotection and chemoprevention. Two commercially available formulations are the crude extract, silymarin, and the semipurified product, silibinin. Silymarin consists of at least seven flavonolignans, of which the most prevalent are the diastereoisomers silybin A and silybin B; silibinin consists only of silybin A and silybin B. Based on a recent clinical study showing an interaction between a silymarin product and the CYP2C9 substrate losartan, the CYP2C9 inhibition properties of silybin A and silybin B and corresponding regioisomers, isosilybin A and isosilybin B, were evaluated using human liver microsomes (HLMs), recombinant CYP2C9 (rCYP2C9) enzymes, and the clinically relevant probe, (S)-warfarin. Silybin B was the most potent inhibitor in HLMs, followed by silybin A, isosilybin B, and isosilybin A (IC(50) of 8.2, 18, 74, and >100 microM, respectively). Next, silybin A and silybin B were selected for further characterization. As with HLMs, silybin B was more potent than silybin A toward rCYP2C9 1 (6.7 versus 12 microM), rCYP2C9 2 (9.3 versus 19 microM), and rCYP2C9 3 (2.4 versus 9.3 microM). Using a matrix of five substrate (1-15 microM) and six inhibitor (1-80 microM) concentrations and HLMs, both diastereoisomers inhibited (S)-warfarin 7-hydroxylation in a manner described best by a mixed-type inhibition model (K(i) values of 4.8 and 10 microM for silybin B and silybin A, respectively). These observations, combined with the high systemic silibinin concentrations (>5-75 microM) achieved in a phase I study involving prostate cancer patients, prompt clinical evaluation of a potential warfarin-milk thistle interaction.
Figures

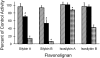
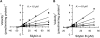


References
-
- Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. (2006) Anticancer potential of silymarin: from bench to bed side. Anticancer Res 26:4457–4498 - PubMed
-
- Bachmann KA, Lewis JD. (2005) Predicting inhibitory drug-drug interactions and evaluating drug interaction reports using inhibition constants. Ann Pharmacother 39:1064–1072 - PubMed
-
- Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. (2005) Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res 65:4448–4457 - PubMed
-
- Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. (2007) A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs 25:139–146 - PubMed
-
- Gazák R, Walterová D, Kren V. (2007) Silybin and silymarin—new and emerging applications in medicine. Curr Med Chem 14:315–338 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

