In vitro osteogenic differentiation of adipose stem cells after lentiviral transduction with green fluorescent protein
- PMID: 19934675
- PMCID: PMC2862472
- DOI: 10.1097/SCS.0b013e3181bf04af
In vitro osteogenic differentiation of adipose stem cells after lentiviral transduction with green fluorescent protein
Abstract
Background: Adipose-derived stem cells (ASCs) have the potential to differentiate into osteogenic cells that can be seeded into scaffolds for tissue engineering for use in craniofacial bone defects. Green fluorescent protein (GFP) has been widely used as a lineage marker for mammalian cells. The use of fluorescent proteins enables cells to be tracked during manipulation such as osteogenic differentiation within three-dimensional scaffolds. The purpose of this study was to examine whether ASCs introduced with GFP-encoding lentivirus vector exhibit adequate GFP fluorescence and whether the expression of GFP interfered with osteogenic differentiation of ASCs in both monolayer and three-dimensional scaffolds in vitro.
Methods: Primary ASCs were harvested from the inguinal fat pad of Sprague Dawley rats. Isolated ASCs were cultured and infected with a lentiviral vector encoding GFP and plated into both monolayers and three-dimensional scaffolds in vitro. The cells were then placed in osteogenic medium. Osteogenic differentiation of the GFP-ASCs was assessed using alizarin red S, alkaline phosphate staining, and immunohistochemistry staining of osteocalcin with quantification of alizarin red S and osteocalcin staining.
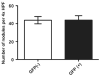
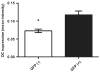
Results: The efficacy of infection of ASCs with a lentiviral vector encoding GFP was high. Cell-cultured GFP-ASCs remained fluorescent over the 8 weeks of the study period. The GFP-ASCs were successfully induced into osteogenic cells both in monolayers and three-dimensional scaffolds. Whereas the quanitification of alizarin red S revealed no difference between osteoinduced ASCs with or without GFP, the quantification of osteocalcin revealed increased staining in the GFP group.
Conclusions: Transduction of isolated ASCs using a lentiviral vector encoding GFP is an effective method for tracing osteoinduced ASCs in vitro. Quantification data showed no decrease in staining of the osteoinduced ASCs.
Figures





Similar articles
-
Comparing the Osteogenic Potential and Bone Regeneration Capacities of Dedifferentiated Fat Cells and Adipose-Derived Stem Cells In Vitro and In Vivo: Application of DFAT Cells Isolated by a Mesh Method.Int J Mol Sci. 2021 Nov 17;22(22):12392. doi: 10.3390/ijms222212392. Int J Mol Sci. 2021. PMID: 34830277 Free PMC article.
-
Osteogenic potential of induced pluripotent stem cells from human adipose-derived stem cells.Stem Cell Res Ther. 2019 Oct 17;10(1):303. doi: 10.1186/s13287-019-1402-y. Stem Cell Res Ther. 2019. PMID: 31623672 Free PMC article.
-
Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice.Biochem Biophys Res Commun. 2004 Jan 23;313(4):871-7. doi: 10.1016/j.bbrc.2003.12.017. Biochem Biophys Res Commun. 2004. PMID: 14706623
-
Leporine-derived adipose precursor cells exhibit in vitro osteogenic potential.J Craniofac Surg. 2008 Mar;19(2):360-8. doi: 10.1097/SCS.0b013e318163e17b. J Craniofac Surg. 2008. PMID: 18362712
-
Osteogenic Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells on Composite Polymeric Scaffolds: A Review.Curr Stem Cell Res Ther. 2025;20(1):33-49. doi: 10.2174/011574888X263333231218065453. Curr Stem Cell Res Ther. 2025. PMID: 38315659 Review.
Cited by
-
Long-term engraftment and angiogenic properties of lentivirally transduced adipose tissue-derived stromal cells.Mol Biotechnol. 2013 May;54(1):13-24. doi: 10.1007/s12033-012-9537-4. Mol Biotechnol. 2013. PMID: 22492300
-
Genetic Modification of Mesenchymal Stem Cells for Neurological Disease Therapy: What Effects Does it Have on Phenotype/Cell Behavior, Determining Their Effectiveness?Mol Diagn Ther. 2020 Dec;24(6):683-702. doi: 10.1007/s40291-020-00491-6. Mol Diagn Ther. 2020. PMID: 32926348 Review.
-
Fibroblast Growth Factor 1-Transfected Adipose-Derived Mesenchymal Stem Cells Promote Angiogenic Proliferation.DNA Cell Biol. 2017 May;36(5):401-412. doi: 10.1089/dna.2016.3546. Epub 2017 Mar 10. DNA Cell Biol. 2017. PMID: 28281780 Free PMC article.
-
Adipose-derived stem cells protect motor neurons and reduce glial activation in both in vitro and in vivo models of ALS.Mol Ther Methods Clin Dev. 2021 Mar 27;21:413-433. doi: 10.1016/j.omtm.2021.03.017. eCollection 2021 Jun 11. Mol Ther Methods Clin Dev. 2021. PMID: 33869658 Free PMC article.
-
Fate of systemically and locally administered adipose-derived mesenchymal stromal cells and their effect on wound healing.Stem Cells Transl Med. 2020 Jan;9(1):131-144. doi: 10.1002/sctm.19-0091. Epub 2019 Oct 15. Stem Cells Transl Med. 2020. PMID: 31613054 Free PMC article.
References
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920. - PubMed
-
- Dudas JR, Marra KG, Cooper GM, et al. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg. 2006;56:543. - PubMed
-
- Kokai LE, Rubin JP, Marra KG. The potential of adipose-derived adult stem cells as a source of neuronal progenitor cells. Plast Reconstr Surg. 2005;116:1453. - PubMed
-
- Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

