Mucins in cancer: function, prognosis and therapy
- PMID: 19935676
- PMCID: PMC2951677
- DOI: 10.1038/nrc2761
Mucins in cancer: function, prognosis and therapy
Abstract
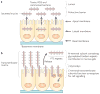
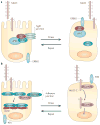
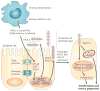
Epithelia are protected from adverse conditions by a mucous barrier. The secreted and transmembrane mucins that constitute the mucous barrier are largely unrecognized as effectors of carcinogenesis. However, both types of mucins are intimately involved in inflammation and cancer. Moreover, diverse human malignancies overexpress transmembrane mucins to exploit their role in signalling cell growth and survival. Mucins have thus been identified as markers of adverse prognosis and as attractive therapeutic targets. Notably, the findings that certain transmembrane mucins induce transformation and promote tumour progression have provided the experimental basis for demonstrating that inhibitors of their function are effective as anti-tumour agents in preclinical models.
Conflict of interest statement
The author declares competing financial interests: see web version for details.
Figures


References
-
- Desseyn JL, et al. Evolutionary history of the 11p15 human mucin gene family. J Mol Evol. 1998;46:102–106. - PubMed
-
- Desseyn JL, Aubert JP, Porchet N, Laine A. Evolution of the large secreted gel-forming mucins. Mol Biol Evol. 2000;17:1175–1184. - PubMed
-
- Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. - PubMed
-
- Herrmann A, et al. Studies on the “insoluble” glycoprotein complex from human colon. Identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J Biol Chem. 1999;274:15828–15836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

