Intranodal immunization with a vaccinia virus encoding multiple antigenic epitopes and costimulatory molecules in metastatic melanoma
- PMID: 19935776
- PMCID: PMC2839441
- DOI: 10.1038/mt.2009.275
Intranodal immunization with a vaccinia virus encoding multiple antigenic epitopes and costimulatory molecules in metastatic melanoma
Abstract
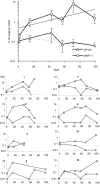
Recombinant vaccinia virus (rVV) encoding tumor-associated antigens (TAAs) and adhesion or costimulatory molecules may represent important immunogenic reagents for cancer immunotherapy. Recently, intranodal (IN) antigen administration was suggested to be more immunogenic than intradermal (ID) vaccination. However, IN rVV administration has not been attempted so far. We used a rVV encoding gp100(280-288), Melan-A/MART-1(27-35) and tyrosinase(1-9) HLA-A0201 restricted epitopes and CD80 and CD86 costimulatory molecules in stage III and IV melanoma patients in a phase 1/2 trial. Of 15 patients initiating treatment, including two cycles of IN immunization, each comprising one rVV administration and three recall injections of the corresponding peptides, accompanied by subcutaneous granulocyte macrophage-colony stimulating factor supplementation, five withdrew due to progressing disease. Of 10 remaining patients seven showed evidence of induction of cytotoxic T lymphocytes (CTLs) directed against at least one epitope under investigation, as detectable by limiting dilution analysis (LDA) of specific precursors and multimer staining. Adverse reactions were mild (National Cancer Institute (NCI) grade 1-2) and mainly represented by fever, skin rashes, and pruritus. These data indicate that IN administration of rVV encoding melanoma-associated epitopes and costimulatory molecules is safe and immunogenic.
Figures


References
-
- Dutoit V, Rubio-Godoy V, Dietrich PY, Quiqueres AL, Schnuriger V, Rimoldi D, et al. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–5856. - PubMed
-
- Schaed SG, Klimek VM, Panageas KS, Musselli CM, Butterworth L, Hwu WJ, et al. T-cell responses against tyrosinase 368-376(370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund's adjuvant, granulocyte macrophage colony-stimulating factor, and QS-21 as immunological adjuvants. Clin Cancer Res. 2002;8:967–972. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

