Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial
- PMID: 19935794
- PMCID: PMC2813746
- DOI: 10.1038/sj.bjc.6605458
Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial
Abstract
Background: Advanced biliary tract carcinoma has a very poor prognosis, with chemotherapy being the mainstay of treatment. Sorafenib, a multikinase inhibitor of VEGFR-2/-3, PDGFR-beta, B-Raf, and C-Raf, has shown to be active in preclinical models of cholangiocarcinoma.
Methods: We conducted a phase II trial of single-agent sorafenib in patients with advanced biliary tract carcinoma. Sorafenib was administered at a dose of 400 mg twice a day. The primary end point was the disease control rate at 12 weeks.
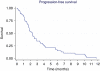
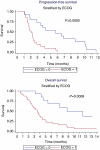
Results: A total of 46 patients were treated. In all, 26 (56%) had received chemotherapy earlier, and 36 patients completed at least 45 days of treatment. In intention-to-treat analysis, the objective response was 2% and the disease control rate at 12 weeks was 32.6%. Progression-free survival (PFS) was 2.3 months (range: 0-12 months), and the median overall survival was 4.4 months (range: 0-22 months). Performance status was significantly related to PFS: median PFS values for ECOG 0 and 1 were 5.7 and 2.1 months, respectively (P=0.0002). The most common toxicities were skin rash (35%) and fatigue (33%), requiring a dose reduction in 22% of patients.
Conclusions: Sorafenib as a single agent has a low activity in cholangiocarcinoma. Patients having a good performance status have a better PFS. The toxicity profile is manageable.
Figures
References
-
- Adjei AA, Christian M, Ivy P (2009) Novel designs and end points for phase II clinical trials. Clin Cancer Res 15: 1866–1872 - PubMed
-
- Beeram M, Patnaik A, Rowinsky EK (2005) RAF a strategic target for therapeutic development against cancer. J Clin Oncol 23: 6771–6790 - PubMed
-
- Berardi R, Scartozzi M, Freddari F, Squadroni M, Santinelli A, Bearzi I, Fabris G, Cascinu S (2006) Biliary tract cancers: molecular profiling as a tool for treatment decisions. A literature review. Cancer Treat Rew 32: 333–347 - PubMed
-
- Clark JW, Meyerhardt JA, Sahani DV, Namasivayam S, Abrams TA, Stuart K, Bhargava P, Blaszkowsky LS, Jain SR, Zhu AX (2007) Phase II study of gemcitabine, oxaliplatin in combination with bevacizumab (GEMOX-B) in patients with unresectable or metastatic biliary tract and gallbladder cancers. J Clin Oncol 25: 18S (Suppl; abstract 4625)
-
- Dal Lago L, D’Hondt V, Awada A (2008) Selected combination therapy with sorafenib: a review of clinical data and perspectives in advanced solid tumor. Oncologist 13: 845–858 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous