Cellular senescence induced by aberrant MAD2 levels impacts on paclitaxel responsiveness in vitro
- PMID: 19935801
- PMCID: PMC2788249
- DOI: 10.1038/sj.bjc.6605419
Cellular senescence induced by aberrant MAD2 levels impacts on paclitaxel responsiveness in vitro
Erratum in
- Br J Cancer. 2010 Jan 19;102(2):456. Mosetto, M [corrected to Tosetto, M]
Abstract
Background: The mitotic arrest deficiency protein 2 (MAD2) is a key component of the mitotic spindle assembly checkpoint, monitoring accurate chromosomal alignment at the metaphase plate before mitosis. MAD2 also has a function in cellular senescence and in a cell's response to microtubule inhibitory (MI) chemotherapy exemplified by paclitaxel.
Methods: Using an siRNA approach, the impact of MAD2 down-regulation on cellular senescence and paclitaxel responsiveness was investigated. The endpoints of senescence, cell viability, migration, cytokine expression, cell cycle analysis and anaphase bridge scoring were carried out using standard approaches.
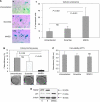
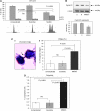
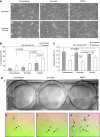
Results: We show that MAD2 down-regulation induces premature senescence in the MCF7 breast epithelial cancer cell line. These MAD2-depleted (MAD2) cells are also significantly replicative incompetent but retain viability. Moreover, they show significantly higher levels of anaphase bridges and polyploidy compared to controls. In addition, these cells secrete higher levels of IL-6 and IL-8 representing key components of the senescence-associated secretory phenotype (SASP) with the ability to impact on neighbouring cells. In support of this, MAD2 cells show enhanced migratory ability. At 72 h after paclitaxel, MAD2 cells show a significant further induction of senescence compared with paclitaxel naive controls. In addition, there are significantly more viable cells in the MAD2 MCF7 cell line after paclitaxel reflecting the observed increase in senescence.
Conclusion: Considering that paclitaxel targets actively dividing cells, these senescent cells will evade cytotoxic kill. In conclusion, compromised MAD2 levels induce a population of senescent cells resistant to paclitaxel.
Figures



References
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life span by introduction of telomerase into normal human cells. Science 279: 349–352 - PubMed
-
- Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB (1999) Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18(34): 4808–4818 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

