Stochastic drift in mitochondrial DNA point mutations: a novel perspective ex silico
- PMID: 19936024
- PMCID: PMC2771766
- DOI: 10.1371/journal.pcbi.1000572
Stochastic drift in mitochondrial DNA point mutations: a novel perspective ex silico
Abstract
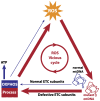
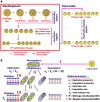
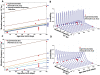
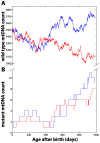
The mitochondrial free radical theory of aging (mFRTA) implicates Reactive Oxygen Species (ROS)-induced mutations of mitochondrial DNA (mtDNA) as a major cause of aging. However, fifty years after its inception, several of its premises are intensely debated. Much of this uncertainty is due to the large range of values in the reported experimental data, for example on oxidative damage and mutational burden in mtDNA. This is in part due to limitations with available measurement technologies. Here we show that sample preparations in some assays necessitating high dilution of DNA (single molecule level) may introduce significant statistical variability. Adding to this complexity is the intrinsically stochastic nature of cellular processes, which manifests in cells from the same tissue harboring varying mutation load. In conjunction, these random elements make the determination of the underlying mutation dynamics extremely challenging. Our in silico stochastic study reveals the effect of coupling the experimental variability and the intrinsic stochasticity of aging process in some of the reported experimental data. We also show that the stochastic nature of a de novo point mutation generated during embryonic development is a major contributor of different mutation burdens in the individuals of mouse population. Analysis of simulation results leads to several new insights on the relevance of mutation stochasticity in the context of dividing tissues and the plausibility of ROS "vicious cycle" hypothesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
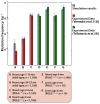
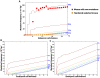
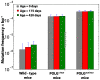
References
-
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. - PubMed
-
- Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS lett. 1991;281(1–2):9–19. - PubMed
-
- Harman D. Free radical theory of aging: dietary implication. Am J Clin Nutr. 1972;25:839–843. - PubMed
-
- Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing - Where do we stand? Front Biosci. 2008;13:6554–6579. - PubMed
-
- Wiesner RJ, Zsurka G, Kunz WS. Mitochondrial DNA damage and the aging process: facts and imaginations. Free Rad Res. 2006;40(12):1284–1294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

